Introduction
In South Korea, two native penaeid species (Fenneropenaeus chinensis and Marsupenaeus japonicus) and an introduced species (Litopenaeus vannamei) are currently cultured, and the majority of farmed production is derived from L. vannamei (Jang et al., 2007, 2009). Shrimp is one of the most economically important aquaculture species in Korea and worldwide; however, many shrimp farms in South Korea have been significantly affected by various pathogens, including white spot syndrome virus (WSSV; Jang et al., 2011a ; Meng et al., 2010). In 2006, 32.9% of the 468 shrimp farms in South Korea had been hit by the WSSV outbreak, although production has recovered since then (Jang et al., 2009). Our previous study demonstrated the positive effects of bioflocs on the growth and immunity of L. vannamei (Kim et al., 2014). Enhancing disease-resistance by improving shrimp immune activity is considered an effective strategy to control diseases in shrimp species including F. chinensis (Huang et al., 2006). The present study investigated some selective immune related genes including: prophenoloxidase1 (proPO1), prophenoloxidase activating enzyme (PPAE), serin proteinase1 (SP1), masquerade-like serine proteinase (Mas), and ras-related nuclear protein (Ran) mRNA expression. Innate immune reactions are the first line of defense against pathogens in vertebrates and invertebrates (Loker et al., 2004). It is initiated by the recognition of pathogen-associated molecular patterns in invading organisms but not in hosts (Janeway & Medzhitov, 2002), which is followed by triggering cellular and humoral responses in hosts (Medzhitov & Janeway, 2000). In invertebrates, cellular responses consist of encapsulation, phagocytosis, and nodule formation while humoral responses include clotting, synthesis of antimicrobial peptides, and activation of the prophenoloxidase (proPO) system (Lee et al., 2004). The proPO activation system plays an important role in defense against pathogens and parasites and during cuticular sclerotization. Activation of the proPO system is triggered by elicitors derived from microbial cell walls, such as lipopolysaccharide, peptidoglycan, and β-1, 3-gulcan (Loker et al., 2004; Medzhitov & Janeway, 2000). Prophenoloxidase activating protein, also referred to as PPAE, is the last protease of the serine protease (SPs) cascade that converts proPO to PO in Manduca sexta (Jiang et al., 2003). SPs constitute one of the largest families of enzymes in the animal kingdom (Shi et al., 2008). SPs engage in many physiological processes via proteolytic cleavage of specific proteins (Rawlings & Alan, 1994). They are generally secreted at extracellular, vesicular, or granular locations, and have extracellular functions (Gorman & Paskewitz, 2001). Several SPs have been cloned from various shrimp: including binding protein of the yellow head virus from Penaeus monodon (Sriphaijit et al., 2007) and pseudo-clip domain containing SPs from L. vannamei hemocytes (Jiménez-Vega et al., 2005). Masquerade-like proteins is one of the SPs, and this protein has been shown to function in granulocyte adhesion, pattern recognition, and opsonization (Huang et al., 2000; Lee & Söderhäll, 2001). To Ran may play roles in L. vannamei immunity against both virial and bacterial infection (Zhang et al., 2019).
Kent et al. (2011) found that in the culture trial of L. vannamei juveniles provided with different sizes of microalgae, the cell size appeared to be a key factor in the feed consumption by the animals. Also, Kim et al. (2015, 2021) reported that the different contributions of biofloc are closely related to the morphological features of the setae on the third maxilliped of each species. Observations of the maxillipeds of the post larvae of L. vannamei, F. chinensis, and M. japonicus revealed that the types and structures of setal affected the biofloc feeding efficiencies of these shrimp species. In particular, F. chinensis and M. japonicus showed a lower response to bioflocs than L. vannamei. The third maxilliped is mouthparts and consists of a large five segmented endopod, a long exopod and an epipod, armed with various types of setae (Alexander & Hindley, 1985; Suthers, 1984). Information generated through studies on the functional morphology of mouthparts and gut could assist in identifying appropriate characteristics of prey or in developing effective formula diets that better meet the nutritional requirements for each life stage of important aquaculture organisms. This approach has been used successfully for various crustaceans, such as, slipper lobsters, spiny lobsters, blue crabs and prawns (Alexander & Hindley, 1985; Cox et al., 2008; Johnston & Alexander, 1999; McConaugha, 2002). The present study investigated the effects of biofloc on the growth, survival rate and immune response of F. chinensis postlarvae and adults and their biofloc feeding efficiencies, as related to the morphological structure of the third maxilliped.
Materials and Methods
A total of 10,000 F. chinensis postlarvae (mean body length 18.3 ± 2.86 mm, mean body weight 29.4 ± 13.53 mg) purchased from a local shrimp hatchery. Animals were acclimated in 1,000 L aquaria of sand filtered, ozone treated and heated to 25°C until start of the study. A total of 400 postlarvae were stocked in 20 L plastic bins. We produced F. chinensis adult (mean body length 106.5 ± 5.77 mm, mean body weight 15.2 ± 3.05 g) form outdoor pond. We were stocked in 200 L tanks of 20 individuals. All experiments were performed with tree replicates in each tank with one air stone each to provide aeration.
The study was conducted at room temperature of 27°C at the West Sea Fisheries Research Institute (WSFRI), National Institute of Fisheries Science (NIFS) Taean, South Korea. 20 L and 200 L rearing tanks were used for each of the three treatments (mean of total suspended solids [TSS] concentrations): biofloc (480 mg/L); mixed (50% BF:50% seawater, 200 mg/L); and seawater (10 mg/L). The rearing tank of each water treatment received recirculated water from the corresponding reservoir at a rate of 50%. Each tank was provided with an air stone at the center of the bottom to keep particles gently suspended and to maintain dissolved oxygen (DO) above 5 mg/L. Seawater for the control biofloc free treatment was sterilized using ozone. The biofloc water source was provided daily from a greenhouse-enclosed intensive shrimp production raceway tank (14-m width × 22-m length × 1.2-m depth) with zero exchange at the experimental station. The mixed water was 50% of seawater and 50% of biofloc water. During the experiments, L. vannamei of approximately 5–10 g were grown with a stocking density of 400–450 shrimp/m2 in the greenhouse raceway tank. Postlarvae were provided with a larval diet (45% crude protein, CJ Feed & Care, Seoul, Korea) and adults were provided with a formulated shrimp feed (40% crude protein, CJ Feed & Care) at three equal daily portions (9:00 a.m., 5:00 p.m., 10:00 p.m.). Water temperature, salinity, pH and DO were measured daily using an YSI 85 meter (YSI, Yellow Springs, OH, USA). Alkalinity, total ammonia-nitrogen (TA-N), nitrite-nitrogen (NO2-N), nitrate-nitrogen (NO3-N) and total bacterial counts were measured every three days in reservoir tanks (Kim et al., 2014).
The number of total bacteria was determined by 4, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO, USA) staining method (Hobbie et al., 1977). Bacterial numbers were calculated as described by Kirchman et al. (1982).
After 14 days, final mean body weights were measured to the nearest 0.1 mg using a scale (BP221S, Sartorius, Goettingen, Germany) and survival rates were calculated at the end of the experiment for each tank.
The names and GenBank accession numbers of the target genes are shown Table 1. At the end of the experiment, three animals were selected from each treatment to be placed in liquid nitrogen and then stored at –80°C until use for RNA extraction. Total RNA was extracted with the RNeasy Mini Kit and then purified with DNase I (Qiagen, Valencia, CA, USA). A TaqMan quantitative reverse transcription polymerase chain reaction (qRT-PCR) technique was used to measure mRNA expression of the six immune-related genes. mRNA expression were measured according to the method of Kim et al. (2014). The relative expression was determined by the comparative threshold cycle method (2-ΔΔCT method, Livak & Schmittgen, 2001).
| Name | Primer sequence (5’ → 3’) | Accession no. & remarks |
|---|---|---|
| proPO1-RT- F | CGAGGGTGGAGAAGCTAGACA | JX644447, present study |
| proPO1-RT- R | CCGGAGTTGCTGATAGTCAGTTT | JX644447, present study |
| proPO1-RT- P | TGGCGCATTCCCATCAAGGACG | JX644447, present study |
| PPAE-RT- F | GGTGACGGTGCCCATCTG | JX644446, present study |
| PPAE-RT- R | CGCACAGCTGCTTGTCGAT | JX644446, present study |
| PPAE-RT- P | CTTGCGACGACGCCTACGAACAGAA | JX644446, present study |
| SP1- RT- F | TCAGGTGGCCCCTTGGT | JX644456, Kim et al., 2014 |
| SP1-RT- R | CAGGACCGTAGGAGACAATGC | JX644456, Kim et al., 2014 |
| SP1-RT- P | CTTGCCGGCACTTTTGGTCCTCC | JX644456, Kim et al., 2014 |
| Mas-RT- F | TGGGATTGCTCCGCACA | JX644444, present study |
| Mas-RT- R | ATTTCCGCCCCTGGAAGAT | JX644444, present study |
| Mas-RT- P | CCCCGTCTGCTTGCCTTCTCAGG | JX644444, present study |
| Ran-RT- F | CCAAGAGAAATTGGGAGGTCTTC | JX644455, Kim et al., 2014 |
| Ran-RT- R | GGGAACATTCTTGTACGTGACTCTAG | JX644455, Kim et al., 2014 |
| Ran-RT- P | ATGGTTACTACATCCAGGCCCACTGTGC | JX644455, Kim et al., 2014 |
| β-actin-RT- F | CCGAGCGTGGCTACACCTT | Present study |
| β-actin-RT- R | GCACAGCTTCTCCTTGATGTCA | Present study |
| β-actin-RT- P | CCACCGCCGAGCGAGAAATCG | Present study |
A hemolymph (0.5 mL) was individually withdrawn from the pericardial cavity of each shrimp into a 3 mL sterile syringe containing 1 mL of anticoagulant solution (Bae et al., 2012). Haemolymphs collected from each of the three samples were pooled in a tube, and then centrifuged. The haemocyte pellet was used for the PO enzyme activity assay. PO activity of hemolymph was determined using the following Jang et al. (2011b) method: The diluted haemolymph was homogenized and hemocyte lysate supernatant (HLS) was collected after centrifuge for 20 min. For a PO measurement, 50 µL of HLS was placed in 96 well micro-plates and incubated for 10min with 50 µL of trypsin (Sigma-Aldrich) at 25°C. Then 50 µL of L-3, 4-dihydroxyphenylalaine (L-dopa; Sigma-Aldrich) was added. After 10 min, a 50 µL homogenized buffer (HB, 10 mM sodium cacodylate, 10 mM CaCl2, pH 7.0) was added, and the PO activity was measured using a microplate reader (PowerWaveXS, BioTek, Winooski, VT, USA) at 490 nm.
All specimens examined in this study were either in the postlarvae or the adult stage. Five individuals of each stage were fixed and examined followed that of Kim et al. (2015). Total endopod length, seta length, seta distance, setule length, setule distance and filtering areas of the third maxilliped endopods were measure and calculated using an equation suggested by Kim et al. (2015). The definition and classification of setal characteristics was generally followed that of Garm (2004a, 2004b).
The results were expressed as the mean ± SD. Significant differences between more than three groups were determined by Stepwise Newman-Keuls test following one-way analysis of variance and results of two parameters results were analyzed using t-tests with 95% confidence level in SPSS 13.0 software.
Results
The water quality parameters in tanks are showed in Table 2. Water temperature was maintained at 28.8°C, DO in a range from 5.5 to 5.7 mg/L and alkalinity, each parameter showing no significant difference between the treatments. On the other hand, other factors including salinity (29.9, 31.7 psu), pH (7.8, 8.4) and inorganic nitrogen (TA-N, NO2-N, NO3-N) concentrations were significantly different among the treatments. In particular, turbidity was 24 times higher in the biofloc water (7 vs. 169.5 mg/L seawater and biofloc, respectively), showing a larger difference between the treatments.
Table 3 shows the total bacterial counts for each treatment and stage. The total number of bacteria in the biofloc water was significantly higher than that in the seawater (Table 3). Also, the seawater and mixed water were significant different between each treatment and stage. But, the biofloc water showed the total bacterial counts ranging from 5.59 × 106 to 1.28 × 107 cells /mL and from 6.79 × 106 to 1.72 × 107 cells/mL for postlarvae and adult, respectively. There was no significant different between the two stages.
Table 4 summarizes the mean final body weight and survival rate of the shrimp for each treatment on the day of the study termination. The final body weight of postlarvae were 51.4 and 58.5 mg for the biofloc water and seawater, respectively, with no significant difference between the two treatments. But the mixed water resulted in the final postlarvae body weight of 90.5 mg, which was significantly higher than those of the other two treatments. And the adults showed similar trends to postlarvae, with the mixed water resulting in the final body weight of 15 g, which was significantly higher than those of the other treatments. Survival rate of postlarvae had no significant difference between the biofloc water and mixed water (32%–36%) but there was significant difference between the biofloc water and the seawater (52%). On the other hand, the survival rate of the adults was not significantly different between all the three treatments.
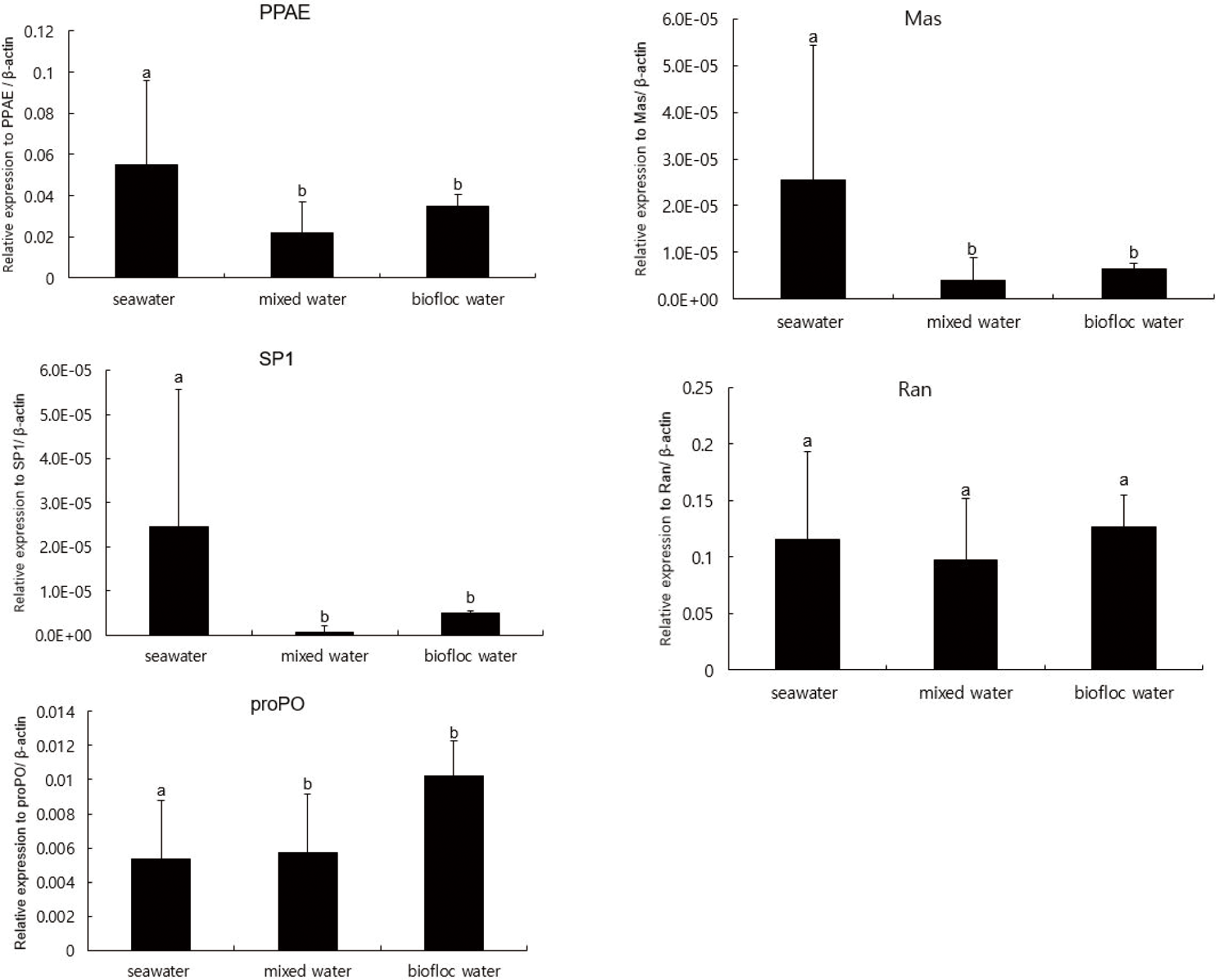
Results of relative mRNA expression in the five immune-related genes are summarized in Fig. 1. The PPAE, SP1, and Mas genes of the biofloc and the mixed water treatment showed significantly lower levels of expression than those of the seawater. But, in the biofloc and the mixed water, the proPO gene expression was significantly higher than that in the seawater.
PO activity was measured in haemocyte of F. chinensis adult. PO activities linearly increased with a higher biofloc concentration. In the biofloc water, PO activity was significantly higher than that of the seawater (Fig. 2).
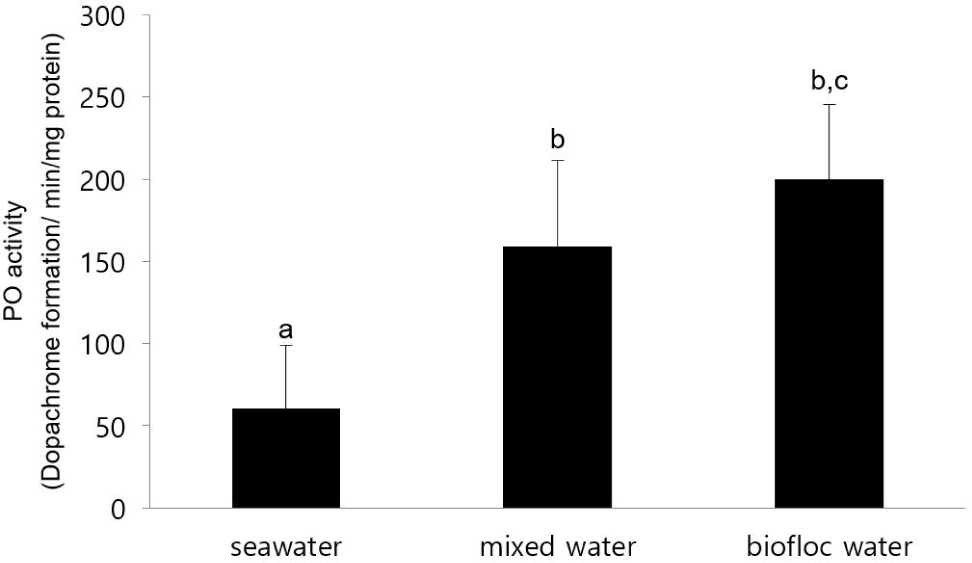
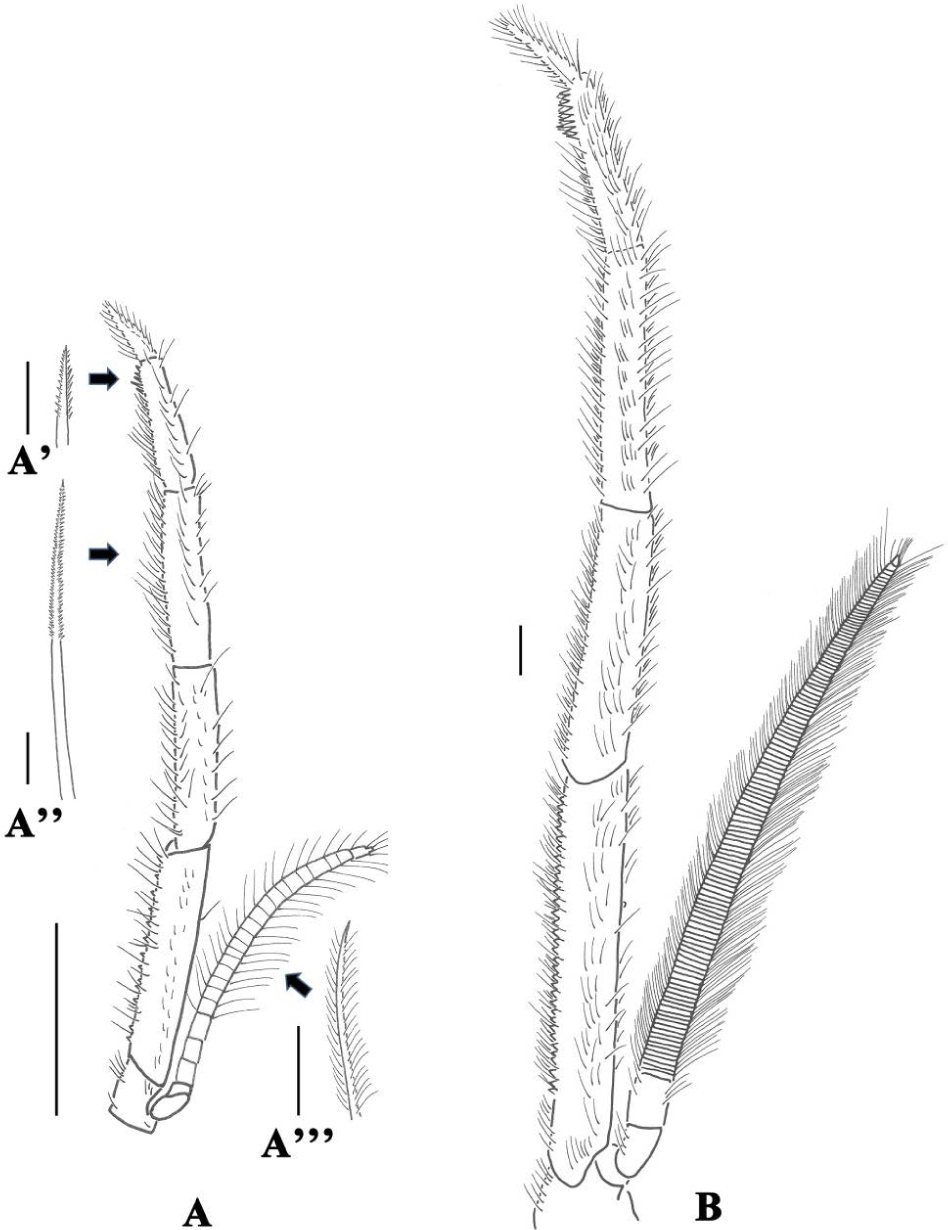
Table 5 summerizes the mean body, total third maxilliped length, seta length, seutla length and distance of the stages. They were sinificantly different between the postlarvae and adult. The seta distance was found to have no significant difference between the stages. The filter area of adult was significantly larger than that of postlarvae. The microscopic drawings and setal description of the endopod segments of the third maxilliped, hereinafter called ‘maxilliped’, in the two stages are provided in Fig. 3 and Table 6, respectively.
Discussions
There were significant differences between the water treatments in some of the water quality parameters. The pH in the biofloc water was lower than that of the seawater. It is to the activities of nitrifying bacteria and CO2 production by biofloc. The concentration of inorganic nitrogen by-products was significantly higher in the biofloc and mixed water due to increased metabolism associated with the rich microbial community in these bins. But, all the water quality parameters were within acceptable ranges reported by other researchers for optimal survival and growth of F. chinensis (Chen et al., 1990; Hu & Lu, 1990). Ray et al. (2010) and Samocha et al. (2007) suggested that the TSS concentrations should range from 460 to 500 mg/L of L. vannamei. But, in the present study, concentrations below the 15 mg/L TSS concentration were found optimal for F. chinensis postlarvae (Tables 2 and 4). A TSS concentration higher than 15 mg/L significantly decreased the survival rate as low as 32%–36%, which is probably because increased TSS concentrations caused by particle accumulation can boost oxygen demand, cause gill fouling in cultured species, suppress beneficial algal growth, and promote blooms of potentially harmful microorganisms (Brune et al., 2003; Hargreaves, 2006; Liltved & Cripps, 1999). In this study, the survival and specific growth rate was significantly different between the treatments. It is immune response relationship to energy consumption their treatments shrimp could not grow. Tyler et al. (2006) said 7.5% increase in energy consumption occurs in response to non-pathogenic immune stimulation. Also, each mean total bacterial count ranged was 9.53E+05 and 2.30E+06 mL–1 in mixed and biofloc water (Table 3). Biofloc stimulates parts of the shrimp immune system (Kim et al., 2014). Crab et al. (2010) said the biofloc technology protected shrimp from pathogen Vibriosis. In contrast, the present study found that expressions of the immune-related genes were lower in the biofloc and mixed water than in the seawater. Finally, too high concentrations of TSS and large amounts of bacteria may give stress to F. chinensis postlarvae. They cannot any effect of growth and immune responses. Our previous study investigated the effect of bioflocs on immune activity and growth rate of L. vannamei postlarvae. As they were cultured in Biofloc Technology-based systems, the shrimp evidently consumed the microbial floc in situ (Ekasari et al., 2014) and (Crab et al., 2012) so that the increases in total haemocyte number and PO activity point in the direction of a stimulatory effect of the (digested) biofloc on shrimp immunity. Kim et al. (2014) clearly showed that the expression levels of proPO, PPAE1 and SP1 genes, which regulate the PO activation systems. The authors expect similar results from this study. But different species bring different results. In this study we using postlarvae other different age we need confirm. In Fig. 1, the PPAE and SP1 genes are significantly high in seawater, and the proPO genes are significantly high in biofloc and mixed water the activity of initial precursor genes such as PPAE, SP1, and Mas appears at the end of the series of experiments, and in the biofloc and mixed waters it is believed that the last proPO activity. On the other hands, in adults, PO activity was significantly higher by two to three times in biofloc and mixed waters, which is consistent with other previous studies (Fig. 2). The survival and growth in postlarvae were higher in seawater, but not at a level that could be said to be better for immune-related gene activity. But, the adults showed high growth in mixed and biofloc water, and high PO activity, an immune indicator (Table 5). This is thought to be related to the increase in filtration area in adults than in postlarvae, and the increase in the efficiency of using floating bioflocs.
It is known that the maxilliped structure of L. vannamei that filter out and feed on floating bioflocs forms a net structure (Kent et al., 2011), and in this study, the filter area formed by the maxilliped structure during the postlarvae is small, and adults form a wide net similar to L.vannamei, resulting in increased filtration and consequently, the bioflocs used in the body contribute to stimulating innate immunity (Kim et al., 2015).
Feeding strategy for a particular species can be evaluated by examining the mouthparts and gut. For example, an increased robustness of the mandibles and complexity of the gastric mill can be correlated to a carnivorous food selection in crustaceans (Kunze & Anderson, 1979). Changes in feeding preference and capability in decapods have been previously associated with changes in the structure and function of indigestive and digestive organs (Cox & Johnston, 2004; Nishida et al., 1990).
Indeed, a range of morphological changes in the mouthparts and gut of Lysmata amboinernsis were observed during the larval development. The potential role of the feeding appendages can be deduced from their form and the type of setae. Accordingly, in the early and late larval stage of L. amboinensis, the maxillae may primarily function for respiration. With the long plumose setae on the scaphognathite creating a current flow over the developing gills. The shorter plumose setae on the endites may act as a net to prevent entry of particles to the branchial chamber (Crain, 1999; Factor, 1978; Farmer, 1974). Our previous study about a different contribution of bioflocs is closely related to the morphological features of the setae on the third maxilliped of these species (Kim et al., 2015).
Conclusions
In this study, the role of the maxilliped seta and setula seems to change as F. chinensis develops from postlarvae to adult. In the adult age, the third maxilliped may act principally as filter feeding in the mouth region, as evidenced by their dense of pappose setae in water column. It means growing a F. chinensis the thrid maxilliped setae was densely and setula was long bring these results, and that a significant increase in the area of filter feeding is also related to the efficiency of using floating biofloc.
The data supporting this is that in addition to innate immune activity in adults, growth and total production shows a significantly higher than in seawater.








