Introduction
Bhutan is located in the southern slopes of eastern Himalayas (Caspari et al., 2006) and is a part of 36 global biodiversity hotspots (Myers et al., 2000). The wide spectrum of ecological conditions ranging from subtropics to alpine meadows has endued the country with rich biological diversity including fishes (Gurung & Thoni, 2015). The country is home to 125 fish species (NBC, 2019) under eight orders (Anguilliformes, Beloniformes, Cypriniformes, Perciformes, Salmoniformes, Siluriformes, Synbranchiformes, and Tertadontiformes) and 24 families (Gurung & Thoni, 2015). Majority of the species belong to orders Cypriniformes and Siluriformes holding high significance in economic landscape, aquaculture, and source of animal protein (Gurung & Thoni, 2015; Nikam et al., 2014; Thai et al., 2007). However, species under other orders and families including Matsacembelidae contribute equally to the economic as well as social livelihood in the country.
Family Mastacembelidae under order Synbranchiformes contains spiny eels with elongated body, having 7–40 depressible well-separated dorsal spines, one to three anal spines, body covered with small scales, lack of pelvic fins and girdles, gill openings on later sides of head, short caudal fin with laterally compressed body towards the caudal region (Arunkumar, 2020; Plamoottil & Abraham, 2014). Globally, Mastacembelelidae consist of three genera (Mastacembelus Scolopi 1777, Macrognathus Lacepède, 1800, SinobdellaKottelat & Lim, 1994) distributed in Africa, the Middle East, Southeast Asia, China, Vietnam, and Laos (Nelson et al., 2016; Yuan et al., 2020). Gurung et al. (2013) recognized two genera of spiny eels (Macrognathus and Mastacemblus) from Bhutan.
Based on current records only three species of spiny eels, namely Macrognathus morehensisArunkumar & Singh, 2000, Macrognathus pancalus Hamilton, 1822, and Mastacembelus armatus Lacepède, 1800 are found in inland aquatic habitats of Bhutan (Gurung & Thoni, 2015; NRDCR & LF, 2020). Arunkumar (2016, 2020) recognized the presence of other species of Macrognathus including Macrognathus aral Schneider and Bloach 1801 from transboundary river drainages of Bhutan. However, M. aral has not been reported from Bhutan, but the authors have recorded a single specimen from Aiechu-kalikhola tributary of Maukhola river at Gelephu town under Sarpang district. Apart from the description of the new record, the authors have also developed a comprehensive key to identify species under Mastacembelidae of Bhutan by examining specimens present in the Ichthyology Laboratory, College of Natural Resources (CNR), Royal University of Bhutan (RUB) and comparing pertinent literature (Jayaram, 2010).
Materials and Methods
A single specimen of M. aral was collected using Electro shocker (Honda GCV 160cc, Honda, Tokyo, Japan) from 26°53.314’ N, 90°31.187’ E on 16 October, 2022. General water parameters were recorded on site using HACH kit (HQ40d, Hach, Loveland, CO, USA) and riparian vegetation was noted. Additionally, other species associated with M. aral in the tributary was also examined. Following Arunkumar (2020) the color of the specimen was noted while it was fresh and euthanized using 0.2 mL of clove oil per 500 mL of water and treated with 10% formalin (Fernandes et al., 2017). The fixed specimen was preserved in 70% ethyl alcohol and catalogued at the Ichthyology Laboratory, CNR, RUB (RUB/CNR/Fish sample/Aiechu-kalikhola/16.10.2022/223).
Identification of the species by comparing it with existing species from Bhutan and adjacent areas was carried out following ; Arunkumar (2020); Biswas et al. (2007); Gurung & Thoni (2015); Jayaram (2010) ; NRDCR & LF (2020); Shrestha (2008); Vishwanath et al. (2007). Methods followed are those of Jayaram (2010). All the measurements were made with digital caliper to nearest 0.1 mm and expressed as percentage of standard length (SL) (Arunkumar, 2020; Duong et al., 2020). Additionally, for diagnostic, spines and fin rays were counted using microscope (Nikon SMZ445, Nikon, Tokyo, Japan). Standard practices of Jayaram (2010); Ng & Tan (2020); Roberts (1980, 1986) were referred.
Results
The initial documentation of a spiny eel, identified as Rhynchobdella aral, originated from Tranquebar, Tamil Nadu, India (Talwar & Jhingran 1991). The species under genera Macroganthus found in the Indian subcontinent has been consistently referred to as M. aculeatus by nearly all authors (Roberts, 1980). However, there are no type specimens for M. aral (Roberts, 1980). Rhynchobdella ocellata was described from Pondicherry, India which was later considered as secondary junior homonym of M. ocellatus (Kottelat, 2013). Subsequently, a new species of spiny eel (R. ocillata) was described from Tenasseri, Myanmar but was considered incorrect subsequent spelling of M. ocellatus (Kottelat, 2013). Hora (1921) reported a new species of Mastacemblid (R. dhanashorii) from Manipur, India, with the only known holotype which was later declared as an ambiguous synonym of M. aral. Malhotra & Dutta (1975) reported a new species of spiny eel (M. jammuensis) from Jammu, India but was declared as a synonym of M. aral (Talwar & Jhingran, 1991). The current status of the species is valid as M. aral (Arunkumar, 2020).
Body elongated eel like, presence of paired tooth plates along the concave ventral surface of rostrum, snout trilobed, rim of anterior nostril with six finger like fimbriae, gape of mouth narrow, jaws with small pointed teeth, pre-orbital and pre-opercular spines absent or smooth, a distinct band on either side of the body above lateral line which becomes obscure in the post-anal region, presence of depressible dorsal spines, anal fin with three spines, caudal fin rounded, the dorsal fin originate much posterior to the pictorial fin, soft dorsal and soft anal fin partially separated by a notch from rounded caudal fin, presence of four ocelli at dorsal fin base, ocellus at base of caudal fin absent. Body count and measurements are shown in Tables 1 and 2 respectively.
| M. aral | Macrognathus pancalus | Macrognathus morehensis | Mastacembelus armatus | ||
|---|---|---|---|---|---|
| Present study | Jayaram (2010) | Gurung & Thoni (2015) | NRDCR & LF (2020) | Gurung & Thoni (2015) | |
| Dorsal fin rays | XIX, 47 | XVI–XXIII, 47–48 | XXIV–XXVI, 30–42 | XI–XVI, 39–51 | XXXIII–XXXX, 67–82 |
| Anal fin rays | III, 52 | II–III, 44–52 | III, 31–46 | III, 40–54 | III, 67–83 |
| Pectoral fin rays | 19 | 19 | 17–19 | 15–20 | 23 |
| Caudal fin rays | 15 | 15 | 11–13 | 11–14 | 14–17 |
| Rostral plates | 28 | 14–28 | Absent | 8–11 | Devoid |
| Present study (cm) | % of SL | Das et al. (2023) (cm) | |
|---|---|---|---|
| Total length | 23.7 | 15.7–32.5 | |
| Standard length | 22.1 | 14.7–27.2 | |
| Head length | 3.6 | 16.5 | 2.5–4.5 |
| Pre-pectoral length | 4.1 | 18.5 | 2.7–5.0 |
| Pre-dorsal length | 7.5 | 34.0 | 5.9–11.0 |
| Pre-anal length | 12.9 | 58.6 | 9.7–17.9 |
| Base of pectoral length | 0.5 | 2.5 | 0.3–0.6 |
| Base of dorsal length | 8.0 | 36.2 | 8.4–15.6 |
| Base of anal length | 7.9 | 36.0 | 4.8–8.9 |
| Pectoral fin length | 1.3 | 6.2 | 0.9–1.6 |
| Dorsal fin length | 0.9 | 4.2 | 0.5–1.0 |
| Anal fin length | 0.6 | 2.8 | 0.4–0.7 |
| Caudal fin length | 1.6 | 5.2 | 1.0–1.8 |
| Body depth | 2.6 | 11.7 | 1.7–3.5 |
| Pre-orbital length | 1.9 | 5.3 | 1.1–2.0 |
| Post-orbital length | 2.1 | 9.6 | 1.2–2.2 |
| Eye diameter | 0.3 | 1.3 | 0.2–0.3 |
Fin formula: D. XIX, 47; P. 19; A. III, 52; C. 15 (23.71 cm TL, 22.13 cm SL)
Body brownish or greenish, yellowish along the abdomen which becomes lighter below, black shades of caudal, soft dorsal and soft anal fins (Fig. 1).

Currently known to occur only in Aiechu-Kalikhola tributary of Maukhola river under Sarpang district.
Seven water parameters (Table 3) were recorded from the point where the specimen was found. Water temperature (℃), dissolved oxygen (DO) (mg/L), conductivity (S/m), total dissolved solids (mg/L), pH, and salinity (g/kg) were recorded onsite while ammonia (µmol/L) was tested at the Soil Water and Air Testing (SWAT) laboratory, CNR, RUB. The habitat was predominantly subtropical with following floras; Senegalia catechu, Ficus semicordata, Toona ciliate, Bombax ceiba, Ficus racemose, Murraya paniculate, Lantana camara, Urena lobata, Solanum viarum, Chromolaena odorata, Sida acuta, Oxalis corniculate, Borreria latifolia, Piper sp., Synedrella nodiflora, and Cyanotis vaga.
A total of 21 species under 10 families (Table 4) were recorded in the Aiechu-Kalikhola tributary where M. aral was found. The most abundant species was Copper mahseer (Neolissochilus hexagonolepis McClelland, 1839) (N = 12), followed by Hamilton’s barila (Opsarius bendelisis Hamilton, 1807) (N = 8) and Giant Daino (Devario aequipinnatus McClelland, 1839) (N = 7) while Glyptothorax botius (Hamilton, 1822) and Golden mahseer (Tor putitora, Hamilton 1822) were the least abundant species (N = 1 each).
Discussion
The native range of M. aral includes lowland habitats of Indian subcontinent including, Bangladesh, Pakistan, Sri Lanka, Nepal, and Myanmar (Talwar & Jhingran, 1991). In the Indian subcontinent M. aral is uniformly distributed where it holds high significance as food and ornamental fish and is widely used in aquarium trade due to its slender body, attractive color pattern, and playful nature (Abujam et al., 2013; Gupta, 2016). Studies have recognized the presence of M. aral from Northeast India including Assam (Arunkumar, 2016; Dhanze et al., 2018; Gupta, 2016) which shares a close boarder with the current study area making the presence of M. aral highly probable. The preliminary checklist of fishes of Bhutan (Gurung & Thoni, 2015) and fishes of Eastern Bhutan (NRDCR & LF, 2020) have reported M. pancalus and M. morehensis respectively, from the same tributary (Aiechu-Kalikhola). However, there was no confirmation of M. aral from Aiechu-Kalilhola. This study confirms the presence and distribution of M. aral in Aiechu-Kalikhola tributary of Maukhola river in Sarpang district. Additionally, pertinent published literatures have reported the co-occurrence of M. aral and M. pancalus in single river system and basins (Arunkumar, 2020; Talwar & Jhingran, 1991).
Macrognathus aral differs from M. pancalus in a number of features. The most distinguishable taxonomic feature includes the presence of rostral tooth plates in M. aral (Fig. 2) which are absent in M. pancalus (Arunkumar & Singh, 2000), lesser dorsal spines (16–23 vs. 24–26), more soft dorsal fin rays (47–48 vs. 30–42) (Jayaram, 2010), preopercular and pre-orbital spines (absent vs. present) (Talwar & Jhingran, 1991). Similarly, M. aral differs from M. morehensis in many significant taxonomic characters. Data of Arunkumar & Singh (2000) for M. morehensis were used for comparison. It can be easily distinguished from M. morehensis by presence of more rostral plates (14–28 vs. 8–11), more dorsal spines (16–23 vs. 11–16), more caudal fin rays (15 vs. 11–14), pattern of band (longitudinal stripes along entire length vs. 20–25 transverse dark band), and ocellus at the base of caudal fin (absent vs. present).
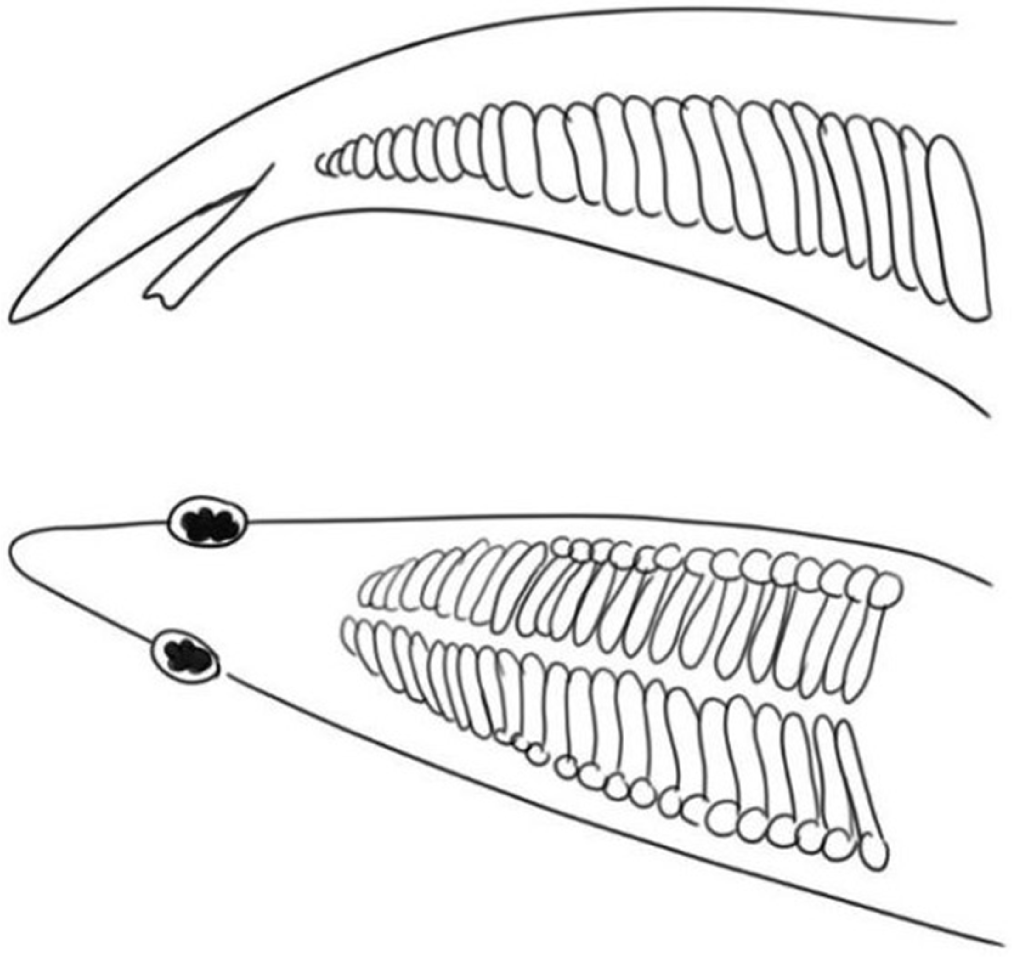
Dorsal fin spines less than 33 (11–26), snout trilobed, ventral surface of rostrum with tooth plates. Rim of anterior tubular nostril with six finger-like projection (Fig. 3A)………………………………………………Macrognathus
Dorsal fin spines 33 or more, no tooth plats in rostrum. Rim of anterior tubular nostrils with two broad-based flaps (Fig. 3B)…..………………………………………….Mastacembelus

1. Concave ventral surface of rostrum with paired tooth plates………………….………………………………………2
Rostrum globous, without tooth plates………………………3
2. Rostral tooth plates 8–11, Dorsal fin spines 11–16………………………………………….……………………M. morehensis
Rostral tooth plates 14–28, Dorsal fin spines 19–23…………………….…………………………………………………M. aral
3. Caudal fin with 11–13 rays...…………………………………4
4. Dorsal fin spines 24–26 and 30–42 soft rays………M. pancalus







