Introduction
Oreochromis niloticus is exotic fish types introduced to many lakes and reservoirs worldwide for aquaculture and fisheries (Vasconcelos et al., 2018). Ethiopia frequently uses the fish species O. niloticus for commercial fishing (Wagaw et al., 2022). Most of Ethiopia’s inland waters are home to the species, which makes up 60% of the fishery’s catch (Getahun, 2007). It is extensively distributed across Ethiopia’s different water bodies and provides significantly to the country’s economy.
Because it serves as the foundation for effective fisheries management plans, an investigation into the diet of fish species is essential (Hussian et al., 2019). The success of fish farming is thought to be greatly improved by determining the feeding habits of fish in a particular ecosystem (Tomojiri et al., 2019).
Investigation in the diet behaviors of fish species in freshwater ecosystem is an ongoing research area because it serves as the foundation for creating effective fisheries management programs for fish capure and culture (Kariman et al., 2009). O. niloticus fry were introduced into Lake Tinishu Abaya in 1997 to promote fisheries and provide the surroundings with low-cost fish protein. Determining the feeding behavior can serve as a guide for stock management of the Lake Tinishu Abaya. To develop a suitable fisheries management approach, it was the purpose of this study to explore the diet behaviour of O. niloticus in Lake Tinishu Abaya.
Materials and Methods
This study was conducted in Lake Tinishu Abaya found in the Ethiopian’s rift valley lakes basin. The lake is placed nearly 160 kilometers far from Addis Ababa (the capital city of Ethiopia). Lake Tinishu Abaya is situated at 1,835 m above sea level. It is located at latitudes 7°29’03.65” N and 38°03’17.79” E of latitude. The lake is relatively small, covering 1,253 hectares of land (Kassahun et al., 2011). The depth of the lake was ranged from 2.9 m to a maximum of 3.7 m. As the depths of the lake indicate, Lake Tinishu Abaya is shallow and productive (Enawgaw & Lemma, 2018). The eastern shore of the lake’s landscape defines the shape of the lake - oval shape (Fig. 1). Lake Tinishu Abaya is fed by two relatively big rivers–River Dacha (in the northern gate) and River Bobodo (in the southern gate). There is another river, river Badober that uses as an outlet of the lake, especially during heavy rainy period (July and August).
There are aquatic macrophytes in and around Lake Tinishu Abaya. Some are include Cyprus sp., Persicaria sp., and Lugonia sp. Most these species of macrophyte habitats are dominantly found in the western coastal region of the lake. This side is relatively protected from human impairment compared to the other corners of the lake. In the eastern corner, there is evident of heavy anthropogenic effect and the site is sparkly coved by few species of macrophytes. The macrophytes experience an increase in coverage during the rainy time (July to August). Some of the macrophyte species, however, become degraded throughout the dry period (January to April).
Lake Tinishu Abaya is a source of income and supports varieties of socio-economic activities, including fish production, irrigation, domestic use, animal watering, etc. The lake is an essential habitat for birds and other aquatic microorganisms. Lake Tinishu Abaya and its watershed are also home to different activities, including crop cultivation. There are fish species in Lake Tinishu Abaya that are significant economically. Tilapia zilli and Barbus fish species are native to the lake. O. niloticus was stocked into Lake Tinishu Abaya in 1997 (Kassahun et al., 2011).
Fish samples were collected using stretched gill net (0.06 m, 0.08 m, 0.1 m, and 0.12 cm meshes sizes). The sample was collected seasonally (dry months: March–May; wet months: July to September). Fingerlings were collected using beach seine (0.04 m mesh size) from the lake’s coastal area. Fish samples were primarily taken from two predetermined sites (one from the middle of the lake water and another one from the littoral region of the lake). The middle of the lake water is the open area of the lake and the site was thought to be pristine because it had experienced relatively little human interference. But because of heavy human involvement in the watershed, the littoral region was relatively exposed to entering high waste.
The collected fish were perpendicularly dissected using dissecting scissors, and each fish’s gut was preserved in the field using a 5% formalin solution in labeled sampling bottles. In the laboratory, the gut content was observed using a dissecting microscope and a compound microscope (100 × magnifications). The relative importance of the different food items identified in the gut contents was determined by the frequency of occurrence (Tesfaye et al., 2020) and volumetric methods (Camera et al., 2014). The significance of various foods for various size classes was ascertained by categorizing the fish in to four size classes. The first group was juveniles-size class having total length of less than 10.0 cm. The second groups were pre-adult stage fish-size class having total length of 10.0 to 19.9 cm. The third size groups were the adult stage-size class having total length of 20.0 to 29.9 cm. The fourth groups were the fish having total length of above 30.0 cm. These size classes of the fish were post adult stage. The mean percentage amounts (in terms of volume) of diet composition for all size categories were then calculated.
Mann-Whitney (non-parameter) tests were employed to determine the seasonal variations in the volume contribution of diet items in the fish’s gut, respectively. Schoener diet overlap index was used to determine the dietary overlap between different length classes of fishes using the following formula (Tesfaye et al., 2020).
Where, pxi: diet item (i) percentage in eaten by size class x; pyi: diet item (i) percentage in eaten by size class y; n: aggregate (sum) of diet items; α: diet overlap proportion between size classes x and y. When the value of the index exceeds 0.60, the overlap is typically thought to be biologically significant.
Results
In the study, 428 fish gut were collected throughout the study. Of them, the majority (n = 373, 87%) were found with one or more food items. The remaining 55 (nearly 13%), were with no food item-empty gut (Table 1). The result showed that phytoplankton and macrophytes were compromised the highest component of the diet of the study fish. Together, they comprised about 65% of the total volume of the food items. Detritus and zooplankton were another desired food items of O. niloticus next to phytoplankton and macrophytes. The former two animal origin food items (detritus and zooplankton) together accounted about 27% of the total food item in the gut content analyzed. The investigation found that the main food sources in the fish gut were typically phytoplankton, detritus, and zooplankton. The other food items (such as insects, nematodes, fish scales, and ostracods) were less consumed by the fish in the study lake (Table 2).
Cynabacteria group of phytoplankton like that of Microcyctis sp. and Anabaena sp., Chlorophyta such as Pediastum sp. and Cosmarium sp., and Bacillariophyta particularly Fragilaria sp., Navicula sp., and Cyclotella sp. were the most desired food items frequently consumed by O. niloticus in the study area. The three phytoplankton groups (i.e., Cyanobacteria, Chlorophyta, and Bacillariophyta) aggregately comprised about 35% of the total volume of the gut content.
The greatest noteworthy sets of animal origin in the diet of O. niloticus were small-bodied rotifers, such as the Branchionus, Keratella, and Filinia species. Rotifers were encountered 67.3% of the time. However, only 6.25% of the total food items were represented by it in terms of volume. Alongside rotifers, the two zooplankton species with large bodies—Crustaceans—accounted a modest quantity to the foodstuff. Crustaceans (i.e., the large-bodied copepods and cladocerans) were collectively occurred 80% of the frequency in the fish diet.
O. niloticus consumed a diet that varied significantly between sampling seasons (Table 2). There were noticeable differences between the two seasons in the volumetric contributions of detritus, macrophytes, and phytoplankton (U-test; p < 0.01). The fish gut was heavily dominated by phytoplankton during the dry period. They made up 56.5% of all food items and were present in 98.5% of the gut’s total volume. However, during the rainy season, their share of the total volume decreased to 32.9%.
Compared to the wet season, macrophytes contributed less during the dry season. Only 20.2% of the gut contents and 14% of the overall foodstuff contained macrophytes in the dry season. However, they were presented in 73% of gut contents and made up 30.6% of all food items in the rainy time. Detritus was O. niloticus’s second most important food source in the wet time. It occurred in 57% and 84% in the dry and wet seasons, respectively.
Similar to macrophytes and detritus, the plant origin foodstuff, the wealth of zooplankton origin food item in the fish gut was greater in the rainy time compared to that of found in the dry months. In the former and later season, they appeared in 52% and 82% of the gut, respectively. Zooplankton made up only 3% of the total volume in the driest period and 20% in the rainiest time. In 8.2% of the gut contents looked at, the involvement of insects in the dry period was relatively low when comparing the two seasons. But it represented a sizable portion (nearly 20%) of the total foodstuff in terms of volume. In 28% of the guts analyzed during the rainy months, insects played a relatively significant role. Volumetrically, the contribution during the rainy season was negligible (0.8%). Nematodes and ostracods made up a larger portion of O. niloticus’ diet during the wet season than they did during the dry. The composition of fish scales was the exact opposite. In the dry and wet months, respectively, ostracods made up less than 10% and nearly 20% of the gut content of the fish diet. Nematodes and fish scales generally made minimal contributions.
The significance of the variation in food item differences between sizes classes are shown in Fig. 2. The gut contents of O. niloticus were found to have significant diet overlap in all size classes (α > 0.6), except the juvenile (size class having a total length of less than 10.0 cm) and the pre-adult stage (fish-size class having a total length of 10.0 to 19.9 cm) (α < 0.6). Between the first sizes class of juvenile fishes, defined as those with a total length of less than 10.0 cm, and the third size class of adult stage fishes, defined as those with a total length of 20.0 to 29.9 cm, the α value was 0.556. This demonstrated there was significant food overlap between juveniles and adults, with the former consuming a lot of food sources with an animal origin (particularly zooplankton) and the latter consuming a lot of food sources with a plant origin (dominantly phytoplankton and macrophtes). Similar to this, there was a significant difference in food items with α value of 0.59 between the second size class (pre-adult stage fishes: size class having a total length of 10.0 to 19.9 cm) and the fourth size class (post-adult stage fishes: size class having a total length of above 30.0 cm). Compared to the food of animal origin, adults consumed more food of plant origin. The values for size classes of the first (juvenile) and the second (pre-adult states) (0.692), the second (juvenile) and the third (adult) (0.783), and the third adult and the fourth (post-adult) indicate that food items overlapped more as the size classes got smaller (0.661).
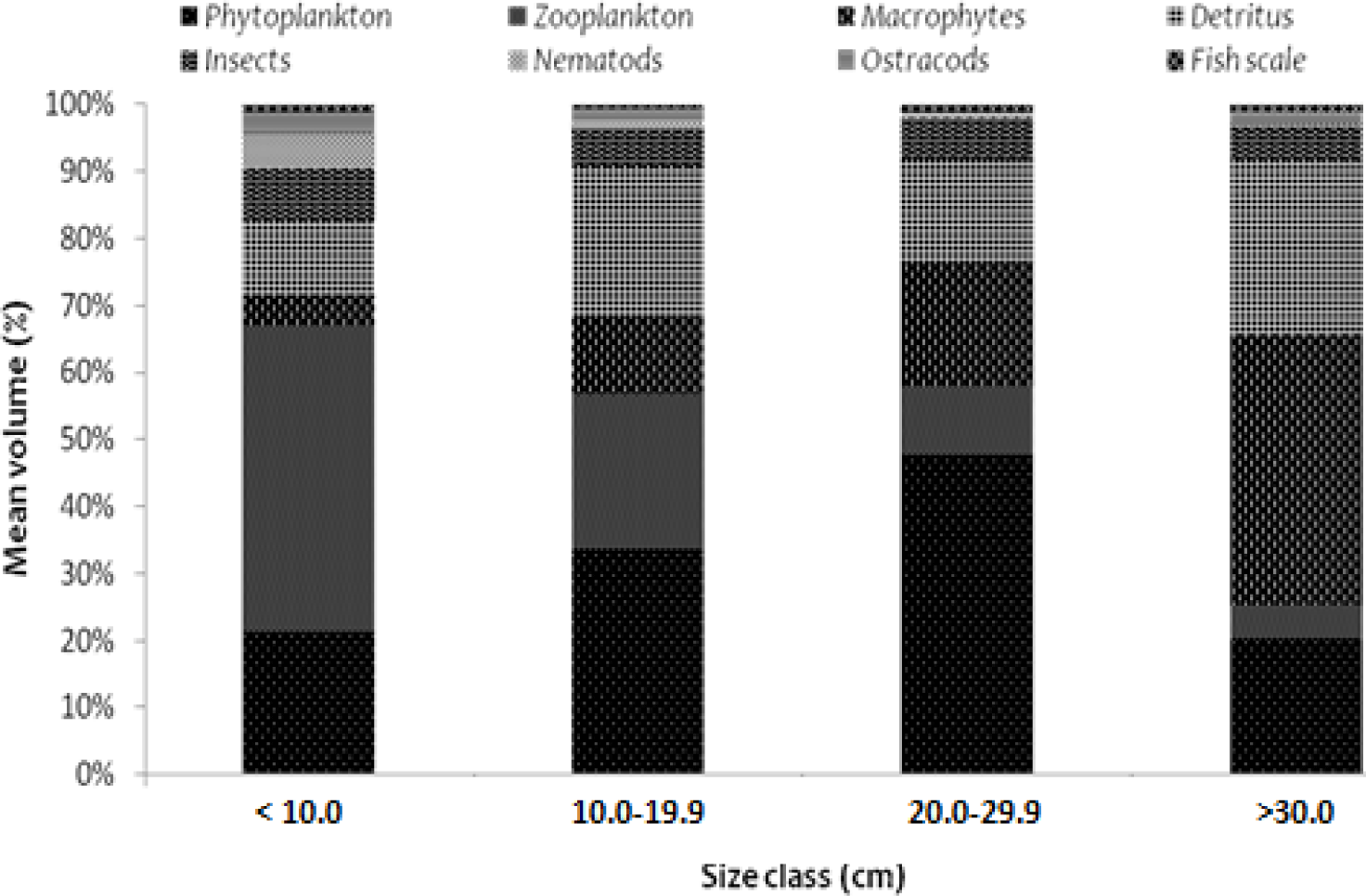
Overall, the dietary overlap between length classes of O. niloticus fish revealed that plant-origin foods like phytoplankton, macrophytes, and detritus became more important with size, while animal-origin foods like zooplankton, insects, and other animal-origin food became less important with fish size. In the study lake, phytoplankton, zooplankton, debris, and macrophytes were the main food sources for O. niloticus in all size classes.
Discussions
O. niloticus, the study subject fish, had a relatively high proportion of empty gut. This notable number of empty stomachs may be attributed to post-harvest digestion or the method of catching the specimens. The empty stomachs of O. niloticus caught with gill nets were also reported by Wagaw et al. (2022) in the salty Lake Shala, Tesfaye et al. (2020) in the Lake Ziway, Assefa & Getahun (2015) in the Lake Hayq, Engdaw et al. (2013) in Koka reservoir, and Oso et al. (2006) in the reservoir at Ero. This might be because the fish struggled to escape the gill nets during the catches, possibly regurgitating or digesting the food items in their stomach.
In this study lake, phytoplankton, zooplankton, macrophytes, detritus, insects, nematodes, ostracods, and fish scales were among the common food sources for the study fish. These are comparable to those that have been reported by numerous authors in various lakes in Ethiopia’s rift valley lakes basin. According to reports, O. niloticus consumes a variety of foods, including phytoplankton, macrophytes, benthic invertebrates, insects, and detritus (Abdulhakim et al., 2015; Negassa & Prabu, 2008; Oso et al., 2006; Teferi et al., 2000).
The current study proved that O. niloticus consumed significant phytoplankton. O. niloticus relied on phytoplankton as its primary source of nutrition usually for which data are available (Elias et al., 2014; Engdaw et al., 2013; Hussian et al., 2019). This indicated that the fish was a phytoplanktivorous or herbivorous feeder. Phytoplanktivorous or herbivorous feeding habits of O. niloticus have also been documented in Ethiopian water bodies like Lake Shala (Wagaw et al., 2022), Lake Chamo (Teferi et al., 2000), Koka Reservoir (Engdaw et al., 2013), and Lake Hayq (Assefa & Getahun, 2015).
Other foods of plant origin (macrophytes and detritus) were also consumed in large amounts besides phytoplankton. The importance of macrophytes and detritus in O. niloticus’ diet was noted by Lake Ziway and Lake Chamo. O. niloticus in Lake Valencia consumed a sizable amount of detritus. Similar interpretations regarding the significance of detritus and macrophyte in various regions of Africa were also offered by several authors (e.g., Shipton et al., 2008). Wagaw et al. (2022) presumed that the O. niloticus fish’s various feeding habits could be explained by variations in the abundance and accessibility of food sources in different water bodies.
There was a seasonal effect in the proportions of various food items found in O. niloticus. A seasonal variation in food types in the diet composition of O. niloticus in the Ethiopian water bodies was also noticed by some prior studies (Assefa & Getahun, 2015; Engdaw et al., 2013). In this study, the proportion of food items that came from macrophytes, zooplankton, and debris in the gut content was higher in the wet season than in the dry season. However, the volumetric contribution of phytoplankton was greater during a dry season compared to a wet season, which is in line with studies by Assefa & Getahun (2015), Kebede et al. (2018), and Wagaw et al. (2022) that claimed phytoplankton is the most significant food source consumed during the dry season.
This agrees with research done by other researchers in the lakes of the Ethiopian rift valley. Some Ethiopian rift valley lakes, including Hawassa, Ziway, and Chamo (Teferi et al., 2000), Abaya (Elias et al., 2014), Langano (Kebede et al., 2018) and the country’s largest lake, Lake Tana, have been found to have a distinct seasonal succession of phytoplankton. O. niloticus primarily consumed phytoplankton during the dry season usually valley lakes for which data are available, whereas macrophytes played a significant role during the wet season. This result, however, contrasted with that found by Wakjira (2013), who found that O. niloticus consumed more phytoplankton in the rainy period in Ethiopia’s Gilgel Gibe I Reservoir.
The differences in the microhabitats occupied by the fish before capture may help to partially explain the composition variations and the relative seasonal contributions of food items. Fish migrate to shallower lake areas during the rainy season to spawn and remain there for extended periods while feeding on macrophytes. The lake’s turbidity may increase and the water level may fluctuate due to high flooding from the catchment during the rainy season. This primarily reduces light that enters the lake, which affects the amount and rate of growth of phytoplankton during the wet season.
The fish move further into the open water and primarily feed on suspended phytoplankton during the dry seasons as the water recedes away from the vegetation. Fish spend the majority of their time feeding in the littoral zones during the wet seasons because there are a lot of macrophytes there. The composition of the fish diet in Lake Tinishu Abaya may change because of changes in the fish’s preferred habitat because of water level fluctuations. This occurred in Lake Lake Hayq (Assefa & Getahun, 2015), Lake Ziway (Tesfaye et al., 2020), Lake Koka (Engdaw et al., 2013), and Lake Langano (Kebede et al., 2018).
O. niloticus has been observed to consume a wide range of foods, including phytoplankton, debris, plant matter, chironomids, and zooplankton (Assefa & Getahun, 2015; Elias et al., 2014; Engdaw et al., 2013; Kebede et al., 2018; Kuebutornye et al., 2019; Oso et al., 2006; Tomojiri et al., 2019; Wagaw et al., 2022; Wakjira, 2013). Depending on size, it might have different feeding preferences. This size-based variation in O. niloticus feeding behavior may be brought on by the fish’s increasing energy needs and the development of its morphological and physiological characteristics (Abdulhakim et al., 2015; Bwanika et al., 2004; Njiru et al., 2004).
O. niloticus displayed variations in feeding behavior based on the size in the current study. Fish juveniles (less than 10 cm in total length) eat primarily foods of animal origin, especially zooplankton, as their main source of nutrition. According to a study conducted in Lake Koka, O. niloticus at smaller sizes (less than 10.0 cm in total length) primarily consumed zooplankton, and as fish size increased, this food type sharply decreased (Engdaw et al., 2013). The ontogenetic dietary shift of O. niloticus in Lake Victoria also revealed that for smaller fish with sizes fewer than 5.0 cm in total length and little importance for larger fish with sizes over 10.0 cm, zooplankton was the most essential food source (Njiru et al., 2004). The results follow Negassa & Prabu (2008) and Teferi et al. (2000), which it is shown that zooplankton, play a significant role in the smaller size of O. niolticus. Juveniles are omnivorous, eating phytoplankton, insect larvae, and zooplankton.
Juveniles’ mouth gaps and small guts, which might not be able to support large macrophytes and debris, are two potential causes of their feeding on zooplankton. Filter feeding is impossible energetically due to the small gut volumes. The inability of the body to process large and difficult foods could also be a factor (Elias et al., 2014). When a fish reaches adulthood, it may switch to gulping the water around it to feed. Phytoplankton dominated the diet of fish in the larger size classes in terms of volume. The contribution of phytoplankton decreased while that of macrophytes and detritus increased in the largest size class. The ability of adult fish to digest detritus and macrophytes in their gut and wider mouth gaps compared to juvenile fish could explain the variation in the proportion of various plant materials throughout the fish’s life cycle.
O. niloticus has a high percentage of detritus in its gut, especially in the large size, according to an analysis of their diet (adult). This suggests that the fish is also a bottom browser, which would place it most likely in the lake’s littoral zones. As a fish ages, the food it consumes changes in composition. Although the early stages of the fish consumed foods of animal origin with high nutritional value, particulate feeding on zooplankton cannot satisfy the energy requirements of growing fish (Shipton et al., 2008). The transition of fish from an omnivorous diet to an herbivorous diet is likely due to energy requirements (Negassa & Prabu, 2008).
Fish and zooplankton in most Ethiopian Rift valley lakes are continuously exposed to predator-prey interactions, as described by Golubtsov et al. (2002) for tropical waters. Daphnia barbata and Moina micrura zooplankton were the dominant grazers in this tropical lake. In Lake Tinishu Abaya, the same results have been seen. This has likely reduced these Cladocerans in the no-fish enclosure to a meagre existence with negligible biomass (Assefa & Getahun, 2015; Vasconcelos et al., 2018).
The fish enclosure in Lake Kuriftu was dominated by a variety of rotifers, including M. micrura, which was only present in small individuals this time. The Lake Tinishu Abaya of the current study has recorded a similar occurrence. These observations suggest that the size factor may have been significant in the relationships between vertebrate planktivorous fish and zooplankton, particularly the Cladoceran relationships. This might explain why O. niloticus adults consume less zooplankton than juveniles do.
In Lake Tinishu Abaya, O. niloticus consumes an equal amount of insects across all size categories. Compared to other size categories, it is a better source for fish under 10.0 cm. Nematodes, ostracods, and fish scales—other animal food sources—were also noticed in the gut of O. niloticus during the study, but their abundance decreased as the fish grew older and larger. In the study lake, the contribution of animal sources—zooplankton, insects, nematodes, and ostracods—is typically higher in smaller size fish, whereas the contribution of plant sources—phytoplankton, macrophytes, and parts of detritus—is typically higher in larger size fish.
Conclusion
The significant differences in food items as the fish sizes are highlighted by the ontogenetic dietary shift of O. niloticus in Lake Tinishu Abaya. The importance of phytoplankton, macrophytes, and detritus increased with fish size while that of zooplankton, insects, and other foods of animal origin decreased as the fish size increased. This ontogenetic diet shifts show that O. niloticus is most likely omnivorous at an early stage; a significant portion of its diet comprises zooplankton and phytoplankton. With increased size, it switches to herbivores. Macrophytes, debris, and phytoplankton, which are foods of a plant origin, made up the majority of the diet at the adult stage. Therefore, it can be concluded that O. niloticus in Lake Tinishu Abaya generally has an omnivorous diet.








