Introduction
Oreochromis niloticus is one of the most important fish species in tropical and subtropical freshwater and frequently serves as the basis for commercial fisheries in several African countries (Stauffer et al., 2022). O. niloticus is the best species for aquaculture because of its high environmental tolerance and capacity to consume both formulated and natural feeds (Khanjani et al., 2022; Munguti et al., 2022). O. niloticus is the most widely distributed fish species in Africa, particularly in the water systems of Ethiopia (Golubtsov & Mina, 2003). Several Ethiopian reservoirs essential for artisanal fishing have this species well-established (Tesfaye & Wolff, 2014). This encourages livelihoods and has an extensive socioeconomic impact on the country (Wondie, 2010). Understanding the biology of this species is essential for fisheries management.
Therefore, understanding the knowledge of some biological characteristics of O. niloticus, such as the relationship between length and weight, is crucial for the study of fish ecology (Tesfaye & Wolff, 2014). According to Das et al. (2021) and Huang et al. (2022), fish length-weight relationships can be used to assess fish length and age structures, population dynamics, growth, mortality rate, and overall health. These are essential tools for collecting information on fish health, stock assessments, and population management (Wagaw et al., 2022). The condition factor is used in fisheries science to evaluate the fitness condition of fish (Wagaw et al., 2022; Wagaw et al., 2024). Using the coefficient value generated by length-weight relationship data, the condition factor can also be used as a tool for studying the lifespan of fish (Huang et al., 2022). However, both biotic and abiotic environmental factors have a significant impact on fish health (Wagaw et al., 2022).
Understanding fish reproductive biology is essential for fisheries management and for filling the knowledge gap in fundamental fisheries research (Tesfaye & Wolff, 2014; Wagaw et al., 2022). Reproductive biology information, such as spawning period and fecundity, is essential in the study of fish biology and population dynamics, as well as in the assessment of the reproductive potential of individual fish species (Das et al., 2021). This will enhance the routine evaluation of numerous economically important fish species and advance our understanding of stock health (Tesfaye & Wolff, 2014). Additionally, having access to data on reproductive characteristics and environmental changes may enhance our ability to estimate recruitment and help us comprehend the variations in reproductive output that have been observed (Wagaw et al., 2022). Data on reproduction and fecundity of O. niloticus can provide essential information for efficient resource management (Das et al., 2021; Huang et al., 2022).
Various aspects of O. niloticus biology have been investigated in Ethiopian lakes and reservoirs (Degsera et al., 2020; Kebede et al., 2018; Teame et al., 2018; Wagaw et al., 2022). However, there are unpublished accounts of O. niloticus biology in Ethiopia’s Geray Reservoir. The objective of this study was to examine the morphometric relationships and some aspects of the reproductive biology of O. niloticus as a first attempt to manage this species in this reservoir. These relationships included length-weight, fecundity-length, fecundity-weight, sex ratio, breeding season, and the gonadosomatic index (GSI).
Materials and Methods
This study was conducted in the Geray Reservoir (Fig. 1) in the Amhara National Regional State of Ethiopia. It is located 387 km and 180 km from the capitals of Ethiopia’s Addis Ababa and Bahir Dar, respectively (Tegegnie, 2015) (Fig. 1). The reservoir was constructed in 1983 and is located in Jabi Tehnan Woreda. It is a run-of-river system with a masonry weir that supplies water to a right-bank canal approximately 5.3 km long. According to the Mohammed et al. (2016), the Geray Irrigation System (GIS) covers 618 ha and has a surface area of 10 ha, weir crest height of 4 m, length of 105 m, and 106 m3 of water. The eastern and western portions of the watershed had different vegetation cover types. The western part has little natural cover owing to increased agricultural activity, but the eastern part is covered by local bushes. The area around Geray Reservoir has an annual average temperature and precipitation of 25.75 °C and 1,350 mm, respectively (Tegegnie, 2015). This reservoir is home to several fish species (Mohammed et al., 2016). The most prevalent fish species in the Geray Reservoir are Tilapia randelli, Cyprinus carpio, Varicorhinus beso, Carassius carassius, and Oreochromis niloticus (Mohammed et al., 2016; Tegegnie, 2015).
The study of the length-weight relationship and different aspects of the reproductive biology of O. niloticus was carried out from November 2021 to August 2022. Three sampling sites (Fig. 1) were used for monthly data of fish samples from the Geray Reservoir. Gill nets were employed to catch the fish, and hooks and lines were used to sample the fish in the area near the outflows of the reservoir, where the cast net design had fallen short. Juvenile fish were caught in the shallow area of the reservoir by using a beach seine with a mesh size of 3 mm. For further testing, the caught fish were kept in ice boxes and delivered to the laboratory.
Morphometric measurements were obtained from the left side of the fish body. Total length (TL) was measured to the nearest 0.1 cm and total body weight (TW) was measured to the nearest 0.01 g using a measuring ruler and weight balance, respectively. Gonads were carefully removed from the fish specimens, weighed on a top weight balance, and preserved in 10% neutral formalin for future research. According to Armstrong et al. (2004), the sex of each specimen was noted, and the gonadal developmental phases were categorized.
According to Geffroy & Wedekind (2020), the sex ratio was calculated by comparing the proportion of the two sexes to one another in the fish population.
Where: X = sex ratio; M = number of male fish; F = number of female fish
The difference in population sex ratio was examined using chi-square (X2) analysis.
Where Oi is the observed value and Ei is the expected value.
In accordance with Le Cren (1951), the power function was used to determine the relationship between the TL and TW of O. niloticus.
Where, TW = Total Weight (g), TL = Total Length (cm), ‘a’ and ‘b’ is constant or parameter (Le Cren, 1951).
The length-weight relation assessment of ‘b’ (Murua et al., 2017) was used to calculate the relative condition factor. The condition factor was calculated according to the approaches of Nehemia et al. (2012) using the following formula:
Where: CF = Condition Factor; TW = Total Weight (g); TL = Total Length (cm) and ‘b’ = the value obtained from the Length-Weight equation.
Where, FCF= Fulton Condition Factor, TW = Total Weight (g) and TL= Total Length (cm)
Gonad inspection was used to determine the gonad maturity rank. Following the steps outlined by Armstrong et al. (2004), the maturity status of individual fish specimens was determined and classified into stages I, II, III, IV and V (Table 1). The preserved ovaries were delivered to the biology laboratory at Debre Markos University for additional examination.
The following equation was used to determine the gonadosomatic index (GSI) for each sex of each fish specimen:
Where, GSI = Gonadosomatic Index; GW = Gonad Weight (g); TW = Total Body Weight (g), where the weight of the gonad is the weight of the fresh gonad blotted on absorbing paper.
The gonadosomatic index (GSI) was calculated for monthly sampled fish specimens to determine the fish breeding season in the lake (Peña-Mendoza et al., 2005).
Straight counts of hydrated eggs were used to determine the fecundity (F) of mature eggs in ovaries (Shoko et al., 2015). The ovaries of mature females were weighed; three sub-samples were taken from the front, mid and rear sections of each ovary and weighed. Then the total number of oocytes in each ovary sub sample was proportionally estimated using the equation, F1 = (gonad weight × number of oocytes in the sub-sample)/sub-sample weight (Yeldan & Avsar, 2000). Later, by taking the mean number of three sub-sample fecundities (F1, F2, F3), the total (absolute) fecundity for each female fish was estimated Ftotal = (F1 + F2 + F3)/3. Regression analysis was used to examine the relationship between fecundity, total body length (TL, cm), and total body weight (TW, g). The relationship is represented by the following formula: F = mTLn and F = aTWb, where F = Fecundity; TL = Total Length (cm); TW = Total Weight (g); m, n, a, and b are constants.
A linear regression analysis was performed using the SPSS version 20.0 statistical software on the length-weight relationship data, which included the association between absolute fecundity and the total length, fecundity, and total weight of fish. The Student’s t-test was used to see whether there was any significant variation from the ideal growth value of 3 (isometric) for the ‘b’ value. Using the Chi-square Goodness (X2) with a 5% significance level, statistical differences from the fictitious distribution of sex in a 1:1 ratio were examined. To compare the absolute fecundity, condition factor, and GSI of O. niloticus, one-way analysis of variance (ANOVA) and the non-parametric Kruskal-Wallis test (p = 0.05) were used.
Results
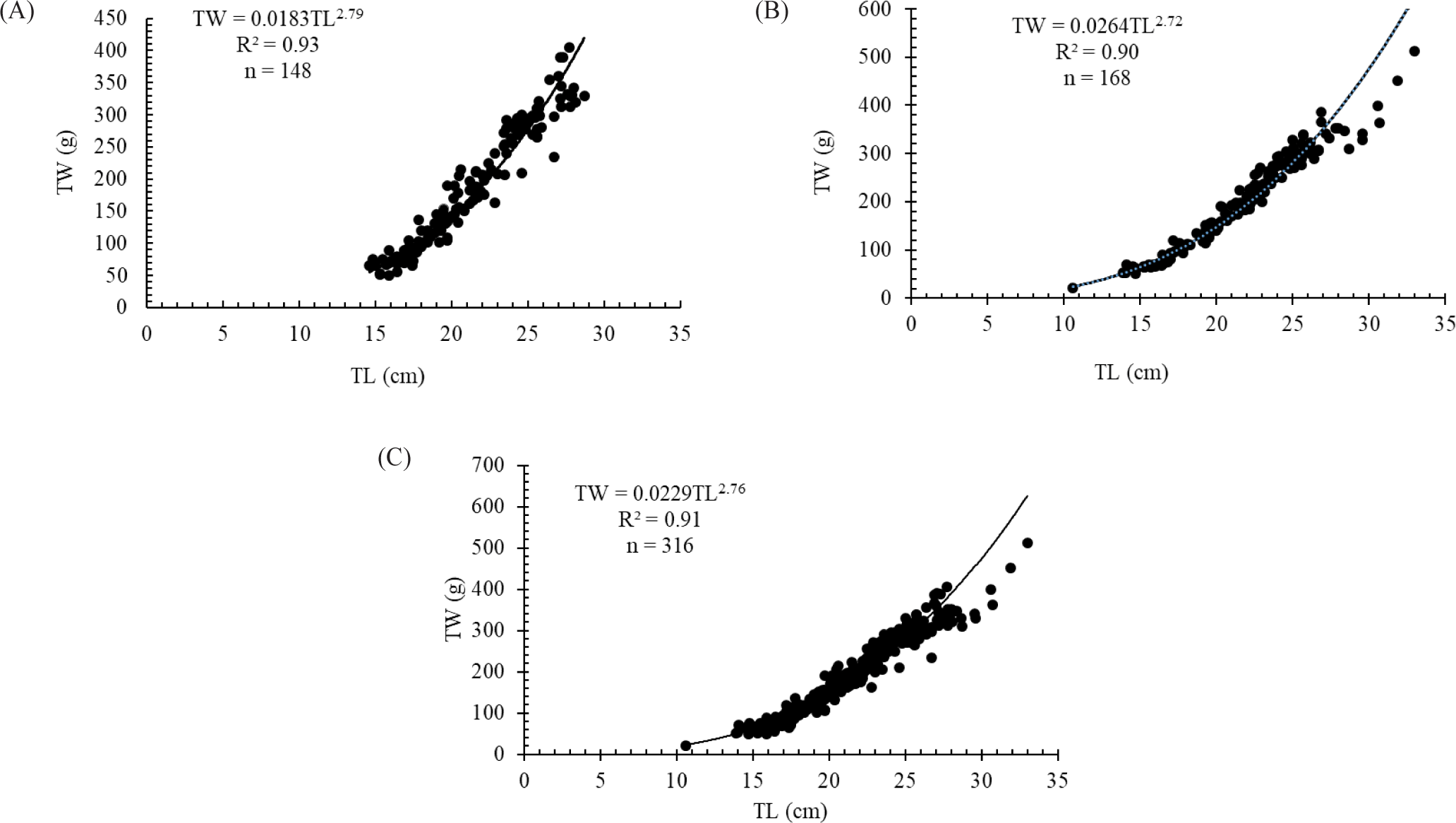
A total of 316 O. niloticus species were collected from different landing sites in the Geray Reservoir. The total length of fish specimens ranged from 10.6 to 33.1 cm. The corresponding total weight ranged from 21.4 and 512.6 g. The length-weight relationship of O. niloticus in the Geray Reservoir was curvilinear and showed a statistically significant (p = 0.0001) difference from the expected isometric growth pattern (Fig. 2). The regression equation for female O. niloticus was TW = 0.0264TL2.72 (r2 = 0.90, n = 168), while for male it was TW = 0.0183TL2.79 (r2 = 0.93, n = 148), and for combined sexes it was TW = 0.0229TL2.76 (r2 = 0.91, n = 316). The combined sex mean value of the length-weight regression coefficient b obtained for O. niloticus (b = 2.76) showed a negative allometric growth pattern (< 3) in the Geray Reservoir.
Of the 316 O. niloticus examined, 148 (46.8%) were males and 168 (53.2%) females had an overall male-to-female ratio of 1:1.14. Generally, females were more abundant than males. However, the Chi-square test indicated that the differences from the hypothetical ratio of 1:1 were not significant (X2 =1.27, p > 0.05).
Table 2 displays the variation in the mean Condition Factor of O. niloticus in Geray Reservoir. The condition factor of O. niloticus varied from 3.34 to 4.40 with a mean of 3.90. The condition factors for males and females ranged from 3.34 to 3.49 and 4.25 to 4.40, respectively (Table 2). The condition factor O. niloticus significantly varied across the categories of sex (p = 0.000), and the value was significantly higher in females (4.33 ± 0.48) than in males (3.41 ± 0.47) (Table 2).
Mean Fulton condition factor (FCF) values of O. niloticus varied from 1.76 to 1.83 for males with a mean value of 1.80. In female O. niloticus, the mean FCF values were in the range of 1.81–1.87 with a mean value of 1.84. For combined sexes of O. niloticus, the mean FCF value was 1.82 ± 0.22 (Table 2). There was no significant variation in the mean Fulton.
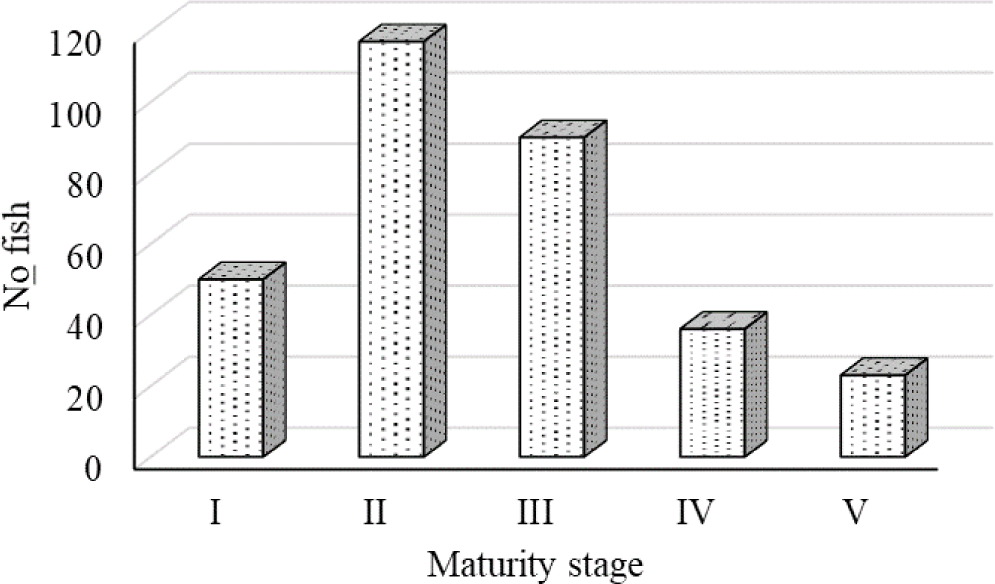
Overall, 316 O. niloticus fish specimens (n = 148, 46.8%) were male and 168 (53.2%) were female. According to these findings, the majority of the fish were in the second stage of maturity (II). As shown in Fig. 3, the percentage of male and female O. niloticus with sexually developed gonads (IV) was 28.5% (n = 90) and 11.4% (n = 36), respectively. Between the stages, the maturity fraction differed considerably (p < 0.05).
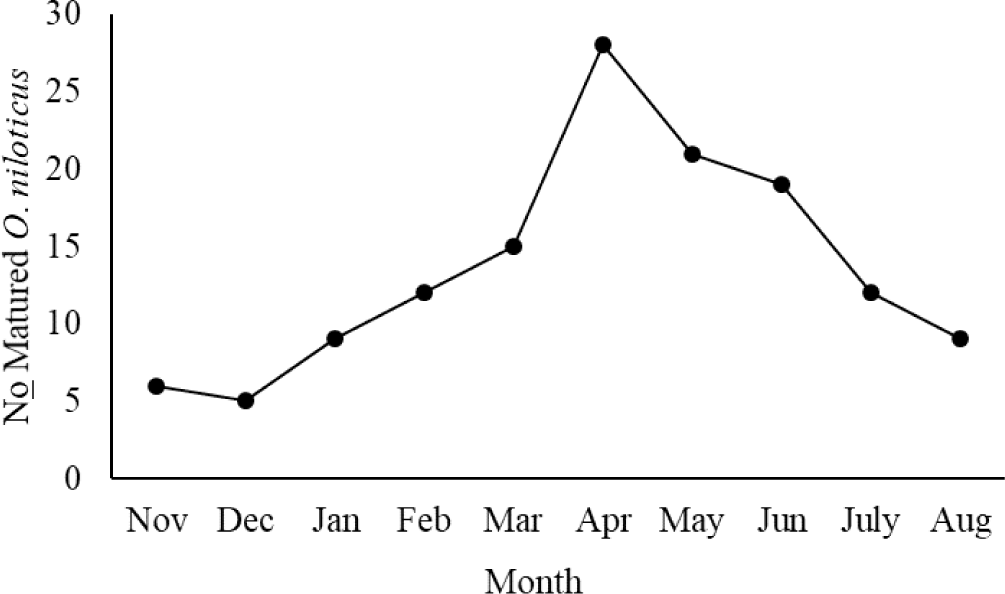
Additionally, seasonal associations of all species for the proportion of fish with sexually developed gonads (stages III to V) were significant (X2, p < 0.05). The wet season (March to June) had a high number of O. niloticus with sexually mature gonads (stages III to V), whereas the dry season (November to February) had a low number of fish species with mature gonads (Fig. 4).
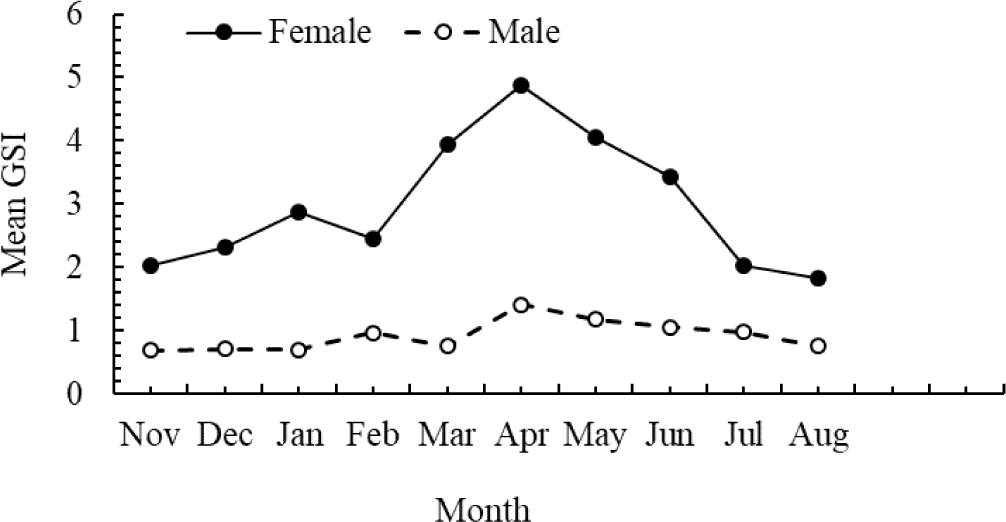
Monthly variations in the GSI showed that both male and female O. niloticus followed nearly the same trend (Fig. 5). In females, the mean monthly GSI values ranged from 1.8 to 4.9 (mean 2.98 ± 1.4) and showed significant temporal variation (p < 0.05). The peak points of female GSI values were observed in March, April, May, and June (Fig. 5). The GSI value of male O. niloticus varied from 0.8 to 1.4 with a mean of 0.92 ± 0.08 (Fig. 5) and did not vary significantly among months (p > 0.05).
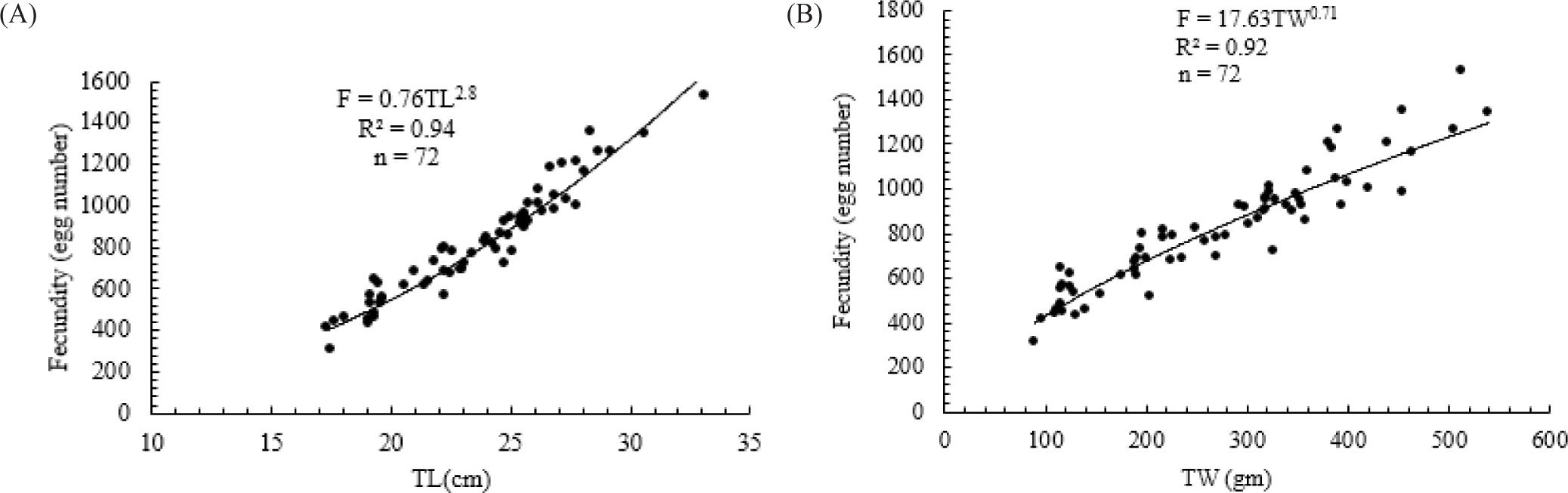
The reproductive potential of O. niloticus was assessed in 72 ripe females. The fish ranged in total length and and total weight from 16.8 to 33.1 cm and 75 to 512.6 g, respectively. According to fish size and weight, the estimated mean fecundity ranged from 279 to 1,528 eggs./fish. In the Geray Reservoir, O. niloticus had a mean fecundity of 1,025 eggs./fish. The total length and weight of O. niloticus in the Geray Reservoir were significantly correlated with absolute fecundity. The relationship equations fitted to the data for fecundity with total length (TL) are described as F = 0.76TL2.8, R2 = 0.94, p < 0.05) (Fig. 6A) and total weight (TW) as F = 17.683TW0.71, R2= 0.92, p < 0.05 (Fig. 6B). The relationships between fecundity and total length and/or total weight were statistically significant (p < 0.05).
Discussion
The results obtained for the LWR showed that O. niloticus in the Geray Reservoir was curvilinear and highly significant. The estimated length weight regression coefficient (b) value of O. niloticus is within the range of b values (2–4) in fishes in general (Tesfaye & Wolff, 2014) and in tropical fishes (b = 2.5–3.5) in particular (Dan-Kishiya, 2013). The slope of the regression line for each sex and combined sex showed that weight increased with length in negative allometry (Fig. 2). For an ideal isometric growth pattern, the ‘b’ value is 3.0, and populations in which the exponent varies from 3.0 show allometric growth (Tesfaye & Wolff, 2014). In the present study the correlation coefficient (r) of O. niloticus showed positive correlation between their total length and total body weight of the fish. This implies that the weight of the fish increases with increasing length (Dan-Kishiya, 2013; Njiru et al., 2006). The negative allometric growth pattern observed in the present study is in agreement with earlier findings on O. niloticus from Lake Beseka (Hirpo, 2013), Lake Hayq (Assefa & Getahun, 2014), and Lake Langeno (Kebede et al., 2018). However, positive allometric growth patterns of O. niloticus were reported by Njiru et al. (2006) and Wagaw et al. (2022) in their study conducted in Lake Victoria and Lake Shala, respectively. These researchers reported the ‘b’ value for O. niloticus as 3.20 and 3.19, respectively. These variations may be due to variations in sex, age, season, and feeding (Tesfaye & Wolff, 2014). In addition, changes in fish shape, physiological conditions, food availability, and life span or growth increments can affect the growth exponent (Wagaw et al., 2022).
The overall sex ratio (Male: Female) for O. niloticus in the Geray Reservoir was 1:1.14; that is, males and females were found in the same number, and the variation from the hypothetical distribution of 1:1 Male: Female was insignificant. Similar findings have been reported for O. niloticus from Lake Shala (Wagaw et al., 2022), Lake Nyamusingiri and Kyasanduka (Bwanika et al., 2004), and the Itapaji Dam (Fagbuaro et al., 2019). However, the predominance of female O. niloticus has been reported in other Ethiopian lakes and reservoirs such as Lake Langeno (Kebede et al., 2018), Lake Hayq (Assefa & Getahun, 2014), and Tekeze Reservoir (Teame et al., 2018). In contrast, Njiru et al. (2006) and Hirpo (2012) reported the dominance of male O. niloticus populations over females in Lake Victoria and Lake Babogaya, respectively. Wagaw et al. (2022) revealed that the sex ratio shows significant variations from the same species in different water bodies, but is usually close to one, and the predominance of sex may differ due to sexual segregation during the spawning period, mortalities in the post-spawning condition, difference in habitat preference, competitive advantage, behavioral variances between the sexes, vulnerability to fishing gear nature, and fishing site (Kebede et al., 2018; Teame et al., 2018).
In the present study, the condition factor for the combined sex was > 1, indicating that fish were above the average condition with good health in the Geray Reservoir. These values were in the range of 2–4 recommended by Dan-Kishiya (2013) as suitable for freshwater fish. The current condition factor value is similar to that in another study of O. niloticus by Wagaw et al. (2022) and Ighwela et al. (2011), who reported that a CF value greater than one signifies that a fish is in good condition. In this study, female O. niloticus was significantly heavier than male CF. The higher CF of female O. niloticus may be attributed to better fat accumulation (Mortuza & Al-Misned, 2013), higher state of maturity, and Gonad Weight (GW) (Wagaw et al., 2022).
The mean values of FCF in this study are consistent with those reported for O. niloticus in Lake Hayq (Assefa & Getahun, 2014), Lake Langano (Kebede et al., 2018), Tekeze Reservoir (Teame et al., 2018), and Lake Shala (Wagaw et al., 2022). In this study, the FCF of O. niloticus values are comparable to those reported in Lake Zeway (Hirpo, 2016), but higher than those reported in Lake Hayq (Assefa & Getahun, 2014), which showed that O. niloticus in the Geray Reservoir had a good body condition. The current mean FCF value is also much lower than that of the same species reported in other Ethiopian water bodies, such as Koka Reservoir and Lake Langeno (Tesfaye & Tadesse, 2008), Lake Babogaya (Hirpo, 2012), and Lake Shala (Wagaw et al., 2022). Variations in the mean FCF obtained in several water bodies could also be credited to the differences in environmental factors, food accessibility and feeding regime, season and water level fluctuation, and water quality. The CF of fish has also been reported to be influenced by many factors, such as spawning period, maturity stage and sex difference (Degsera et al., 2020).
The condition factor reflects fish health (Tesfaye & Wolff, 2014). When determining the period of gonad maturation and monitoring the level of feeding activity of a species to ensure that it makes good use of its source, it provides information when comparing two populations living in specific feeding, density, climate, and other conditions (Ighwela et al., 2011). Fish age, season, and developmental stage can all affect the condition factors (Degsera et al., 2020). Usually, it becomes smaller as the fish grow larger. Although female O. niloticus in the present study had a slightly higher Fulton Condition Factor (FCF) than male O. niloticus, the difference in the Geray Reservoir was not statistically significant (p > 0.05). This agrees with the results of Kebede et al. (2018) and Wagaw et al. (2022) for Lakes Langano and Shala, respectively. According to Tesfaye & Tadesse (2008), both biotic and abiotic factors, such as the feeding schedule and stage of gonadal development, may affect the condition of a species or population over time.
The monthly fluctuations in GSI values and sexual maturity stage in the current study corresponded to seasonal changes in the frequency of O. niloticus with ripe gonads. The GSI findings demonstrated that O. niloticus can breed year-round in Geray Reservoir (Fig. 5). The wet season (March – June) was the peak breeding period for O. niloticus in the Geray Reservoir despite the fact that year-round breeding conditions were recorded. Low seasonal changes in temperature and photoperiod in tropical water bodies are advantageous for spawning at any time of the year (Teame et al., 2018). Therefore, it is possible that breeding of O. niloticus occurred year-round in the Geray Reservoir. In this study, the GSI values for female O. niloticus showed considerable temporal fluctuations, with the peak breeding season occurring between March and June (Fig. 5). This is consistent with reports from several Ethiopian water bodies (Assefa & Getahun, 2014; Kebede et al., 2018; Tesfaye & Tadesse, 2008), coinciding with the start of the rainy season. In contrast, bimodal breeding patterns for the same species have been observed in Tekeze Reservoir (Teame et al., 2018), and Lake Shala (Wagaw et al., 2022). According to Teame et al. (2018) and Wagaw et al. (2022), differences in the aquatic ecosystem’s food supply and water temperature may be the cause of O. niloticus’s varied intensive breeding seasons in various bodies of water.
O. niloticus fecundity (1,025 eggs/fish) in Geray Resetvoir is lower than those found in Lake Victoria (Njiru et al., 2006), Lake Chamo and Koka Reservoir (Endebu et al., 2021). However, the mean absolute fecundity of O. niloticus in the Geray Reservoir was higher than that reported in Lake Hayq (Tessema et al., 2019), Lake Ziway (Endebu et al., 2021), Lake Langen (Kebede et al., 2018), and Lake Shala (Wagaw et al., 2022). This vast range may be explained by the rich supply of natural food resources in the habitat (Wagaw et al., 2022). According to Kebede et al. (2018), fishing pressure results in a typical variation in the mean absolute fecundity of fish species among individuals of the same species in different water bodies. Additionally, variables affecting fish growth and body condition may contribute to variations in fertility (Tessema et al., 2019). According to Peña-Mendoza et al. (2005), fish with less favorable body conditions have a lower fertility capacity.
In the current study, fecundity and total length in O. niloticus were shown to have a curvilinear relationship, but total weight and gonad weight exhibited a linear relationship. Similar results have been recorded for Lake Ziway and Koka Reservoir (Endebu et al., 2021), Lake Langeno (Kebede et al., 2018), and Lake Shala (Wagaw et al., 2022). Fecundity increases proportionally to 2.81–3.96 power of the total length for many tropical freshwater fish species (Tesfaye & Wolff, 2014). According to Degsera et al. (2020), the value of “b” is roughly 3 when fecundity is related to length and roughly 1 when it is related to weight. This biological concept is supported by the fact that O. niloticus fecundity in the Geray Reservoir depends on both total weight and length. Similarly, Kebede et al. (2018) and Wagaw et al. (2022) revealed that the fertility of O. niloticus depends on both total length and weight in Lake Lengeno and Lake Shala, respectively, which are mostly determined by environmental conditions and availability of food in the environment.
Conclusion
In this study, O. niloticus in the Geray Reservoir was studied for length-weight relationships, condition factors, breeding timing, and fecundity. The findings of this study showed that O. niloticus males, females, and combined sexes had negative allometric growth. The results for the condition factor of both males and females are greater than 1, which indicates that most fish in the reservoir are heavy for their respective lengths. However, the Fulton condition factor (FCF) of male and female O. niloticus significantly differs, indicating the necessity for critical investigations to identify the environmental drivers of this variation in order to maximize fishery production. In the present study, O. niloticus spawning in the Geray Reservoir peaked in April and lasted until June. To avoid catching O. niloticus spawning, it is advisable to scale back fishing operations during the peak breeding season. This study also suggests the need for more research into feeding ecology and population parameter analyses, together with other environmental conditions.