Introduction
Particulate matter (PM) is complex mixture includes both organic and inorganic particles, such as dust, pollen, soot, smoke, and liquid droplets (Ghazi & Khadir, 2009) which could cause several diseases (Peng et al., 2008) including the eye (Chen et al., 2016), thus PM has been classified as a class 1 carcinogen in the worldwide (Liu et al., 2022). When the ocular surface is exposed to environmentally toxic substances like PM, the damage rate is higher than that of other internal organs as there is no protective physical barrier (Rauchman et al., 2023). Typical eye diseases caused by PM exposure include conjunctivitis, dry eye disease, and blepharitis (Choi et al., 2019). On the other hand, it is important that eyes being sensory organs could lead to PM-induced complications in the nervous system including the brain (Chua et al., 2020; Wang et al., 2017; Zhu et al., 2019). So, exposure to PM would cause complex disease. In addition, there is no treatment to cure the eye related complications caused by PM exposure and there is a scarcity in therapeutic interventions for treatment of PM-induced eye diseases (Song et al., 2021).
Octopus minor known as long arm octopus is an invertebrate belonging to class Cephalopoda. Its native distribution is in the North Pacific Ocean area including the sea belt of Republic of Korea (Jung et al., 2018). Among the therapeutic compounds isolated from Octopus minor recorded in scientific literature, cardioactive peptides had shown both positive chronotropic and inotropic effects on the heart while synthetic peptides of octopromycin and octominin had shown antibacterial and antifungal activities, respectively (Iwakoshi et al., 2000). Also, cephalopod skin ommochrome pigments are reported to exhibit oxidative stress protection (Lewis et al., 2022). Although much of the physiological activity of octopus has not been fully revealed yet, its excellent antibacterial and antioxidant activities suggest the potential to prevent oxidative stress caused by PM.
The current research was conducted using in vivo zebrafish vertebrate model which is an advantage in vivo model due to the conserved genes with human with cost effective and transparency. Zebrafish display visual responsiveness 72 hours post fertilization (hpf), with a retina similar to humans. The retinal architecture consists of photoreceptor subtypes and cones, resulting in color vision similar to humans (Richardson et al., 2017). In this study, therefore, we evaluated the protective effect of Octopus minor extract (OME) against PM-induced eye injury using zebrafish embryos.
Materials and methods
PM in this study was purchased from national institute of Japan (NIES, Ibaraki, Japan). 2',7'-Dichlorofluorescin diacetate (DCFH2-DA) and tricaine methane sulfonate (MS-222) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemical reagents were used > 99.8% purity levels.
Wild type, AB strain and transgenic Tg (mito:eGFP) adult zebrafish, were obtained from the Fluorescent Reporter Zebrafish Cooperation Center (FRZCC 1072) of Korea University, were maintained at 28.5°C under a 12-h light/12-h dark cycle. Its embryos were collected and raised in egg water (0.75 g CaSO4 and 3 g sea salt in 1 L of pure water). The embryonic stages were expressed in hpf and days post-fertilization (dpf). Experiment was conducted by following Hanseo university ethical guidelines (2022-F-002).
PM stock solution (5 mg/mL) was prepared with egg water. The crude 80% ethanol extract of long arm octopus (Octopus minor) body tissues was received from the National Marine Biodiversity Institute of Korea (MABIK). The extract was dissolved in 100% dimethyl sulfoxide (DMSO, LPS Solution, Daejeon, Korea) to make the stock solution of 20 mg/mL. The 6–7 hpf embryos were place into 24-well plate (SPL Life Sciences, Pocheon, Korea) and treated with OME at a concentration of 20 μg/mL. After 60 min, 300 μg/mL concentration of PM treated into the wells. The co-treatment of OME and PM was continued until at 3 dpf. There were three groups namely; Control (CON) group, PM exposure (300 μg/mL) group, Octopus minor extract (20 μg/mL) + PM (300 μg/mL) (OME + PM) group. Controls were maintained by adding the same concentration of DMSO as in the co-treatment group of OME + PM which had the highest concentration of DMSO among all three groups.
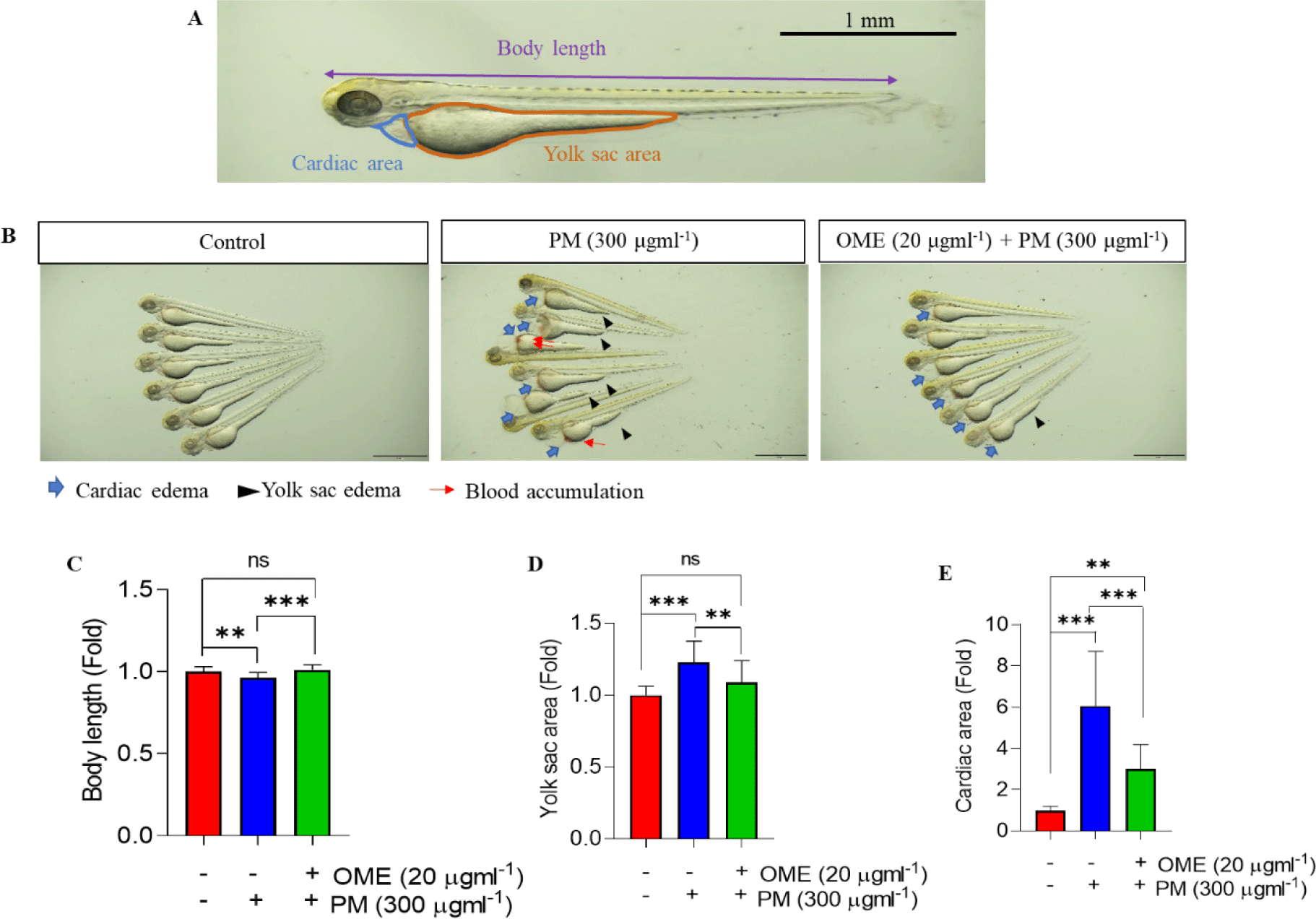
The embryos were observed at 3 dpf after the treatment using a microscope (SZX16, Olympus, Tokyo, Japan) to determine the mortality, heartbeat and malformation. Photographs were taken for 6 embryos in lateral view after anesthetizing with 0.03% tricaine methane sulfonate (MS-222, Sigma-Aldrich). Total body length was marked by drawing a straight line from head to tail while demarcating the cardiac and total yolk sac area in the same way using Olympus cellSens Standard software (Version 3.1, Olympus) and then were quantified with ImageJ 1.53t software (National Institutes of Health, Bethesda, MD, USA). Heartbeats were counted for 3 min under microscope and present as meanbeats value per 30 s.
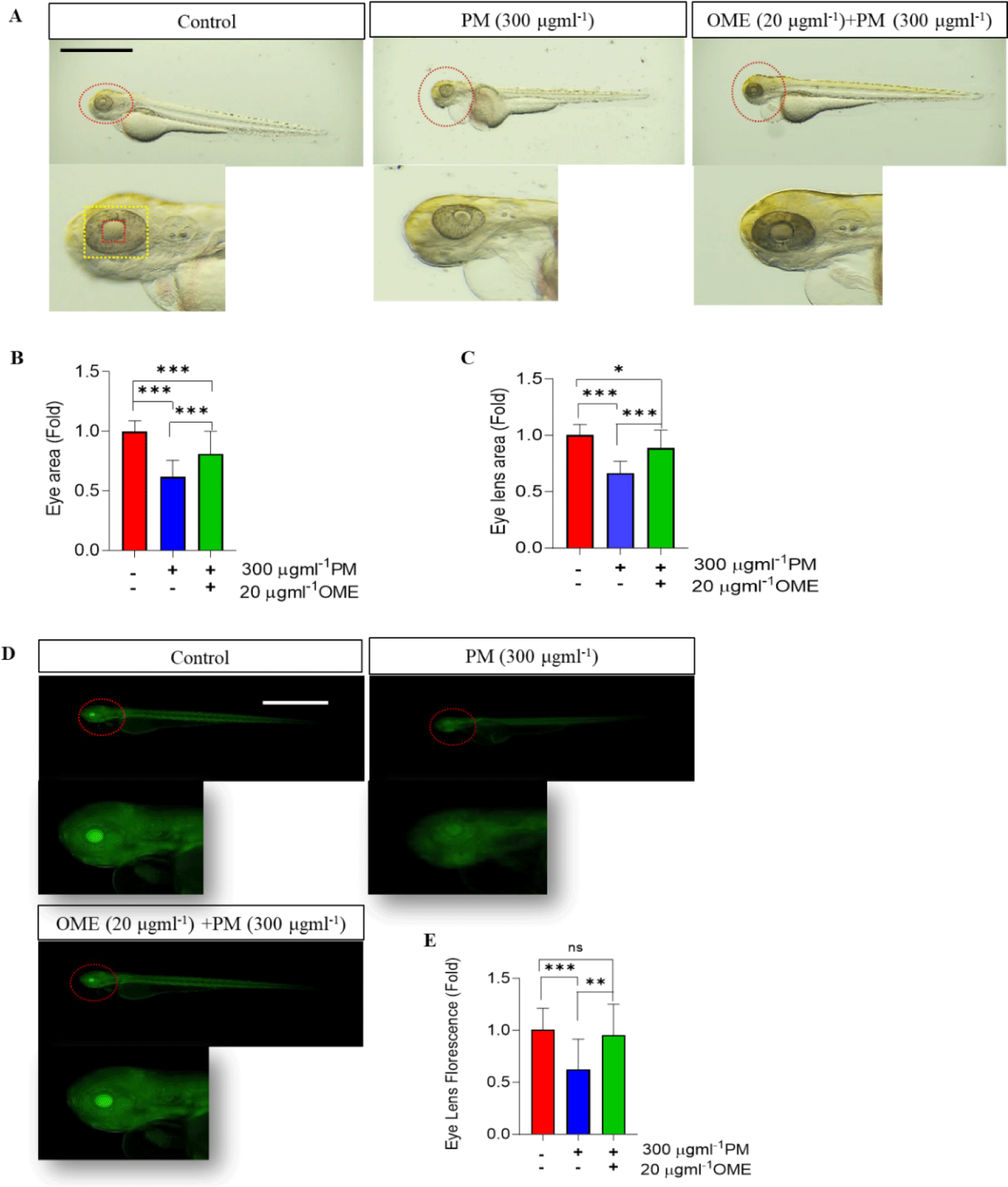
Tg (mito:eGFP) embryos were started to treat with 0.2 µM PTU (Sigma-Aldrich) at 24 hpf and continued until 3 dpf in all experiment groups, in order to clearly visualize the eye and lens margin and eGFP florescence from the embryos. Then embryos were a photographed in the lateral view after anesthetizing with 0.03% tricaine. After anesthetizing with 0.03% tricaine. Eye and lens were demarcated by drawing circular lines using Olympus cellSens Standard software (Version 3.1, Olympus) and then quantify with ImageJ 1.53t software (National Institutes of Health, Bethesda).
Wild type embryos (n = 8–10) from each of the three treatment groups were subjected to RNA extraction in 3 independent experiments. The embryos were obtained at 3 dpf and anesthetized with MS-222 and washed 2–3 times in phosphate-buffered saline (PBS) before subjecting to total RNA extraction using RNAiso Plus (TaKaRa Bio, Shiga, Japan) according to manufacturer’s instructions. The isolated RNA subjected to cDNA synthesis using PrimeScript™ 1st strand cDNA Synthesis Kit (TaKaRa Bio) following manufacturer’s instructions.
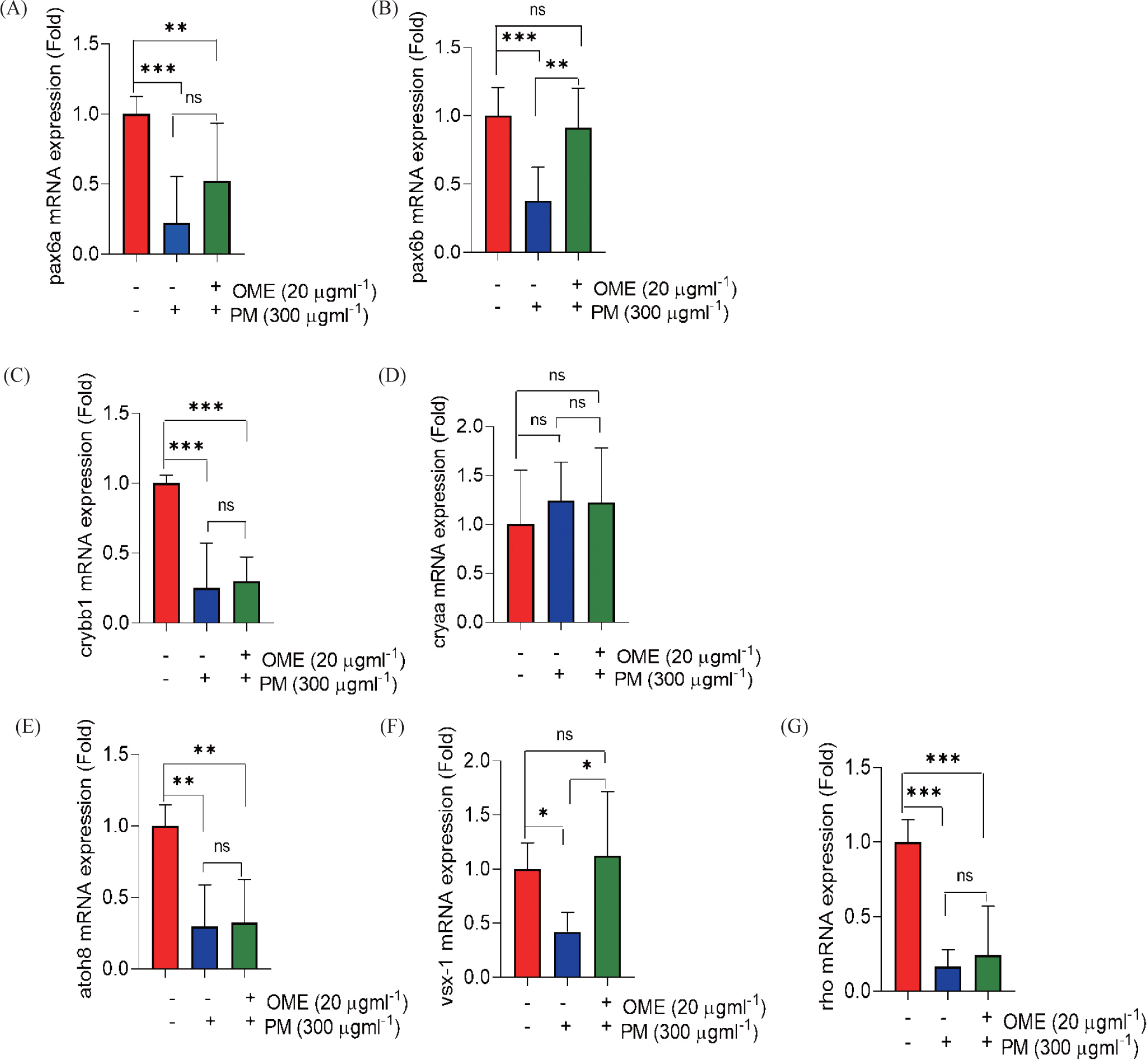
RT- qPCR was performed using SYBR Green (TB Green® Premix Ex Taq™ II [Tli RNaseH Plus], ROX plus, TaKaRa Bio) in Bio-Rad RT-PCR system (Bio-Rad laboratories, Hercules, CA, USA). Relative mRNA expression levels of genes coding proteins having the major functions of eye development and embryogenesis as described in the following section of this paragraph were measured using the cDNA synthesized from the embryos. Crystallin, alpha A (cryaa), crystallin beta B1 (crybb1), paired box (pax) 6a, pax6b, rhodopsin (rho), atonal bHLH transcription factor 8 (atoh8), visual system homeobox (vsx1), catalase (cat), superoxide dismutase 2 (sod 2), nuclear factor erythroid 2 - related factor 2 (nrf2), nuclear factor kappa-light-chain-enhancer of activated B cells (nf-kb), cyclooxygenase-2 (cox-2), tumor necrosis factor alpha (tnf-α), interleukin-1β (il-1β). When performing RT-qPCR of the aforementioned genes, β-actin 1 (actb1) was used as the housekeeping gene. Oligo sequences are listed in Table 1. The primers were designed using the National library of medicine, National Center for Biotechnology Information website (NIH, Bethesda, MD, USA) and web primer design program, Primer3web. The fold changes for relative mRNA expression of each gene were calculated using 2-ΔΔCt method based on threshold cycle (Ct) number. All the relative mRNA expressions were normalized to β-actin 1 (actb1).
The Zebrafish Information Network (ZFIN), University of Oregon, USA is a database of genetic and genomic data for the zebrafish as a model organism.
actb1, actin, beta 1; cryaa, crystallin, alpha A; crybb1, crystallin beta B1; pax6a, paired box 6a; pax6b, paired box 6b; atoh8, atonal bHLH transcription factor 8; vsx1, visual system homeobox 1 homolog, chx10-like; rho, rhodopsin; nrf2, nuclear factor erythroid 2-related factor 2; cat, catalase; sod2, superoxide dismutase 2; il-1β, interleukin 1 beta; tnf-α, tumor necrosis factor alpha.
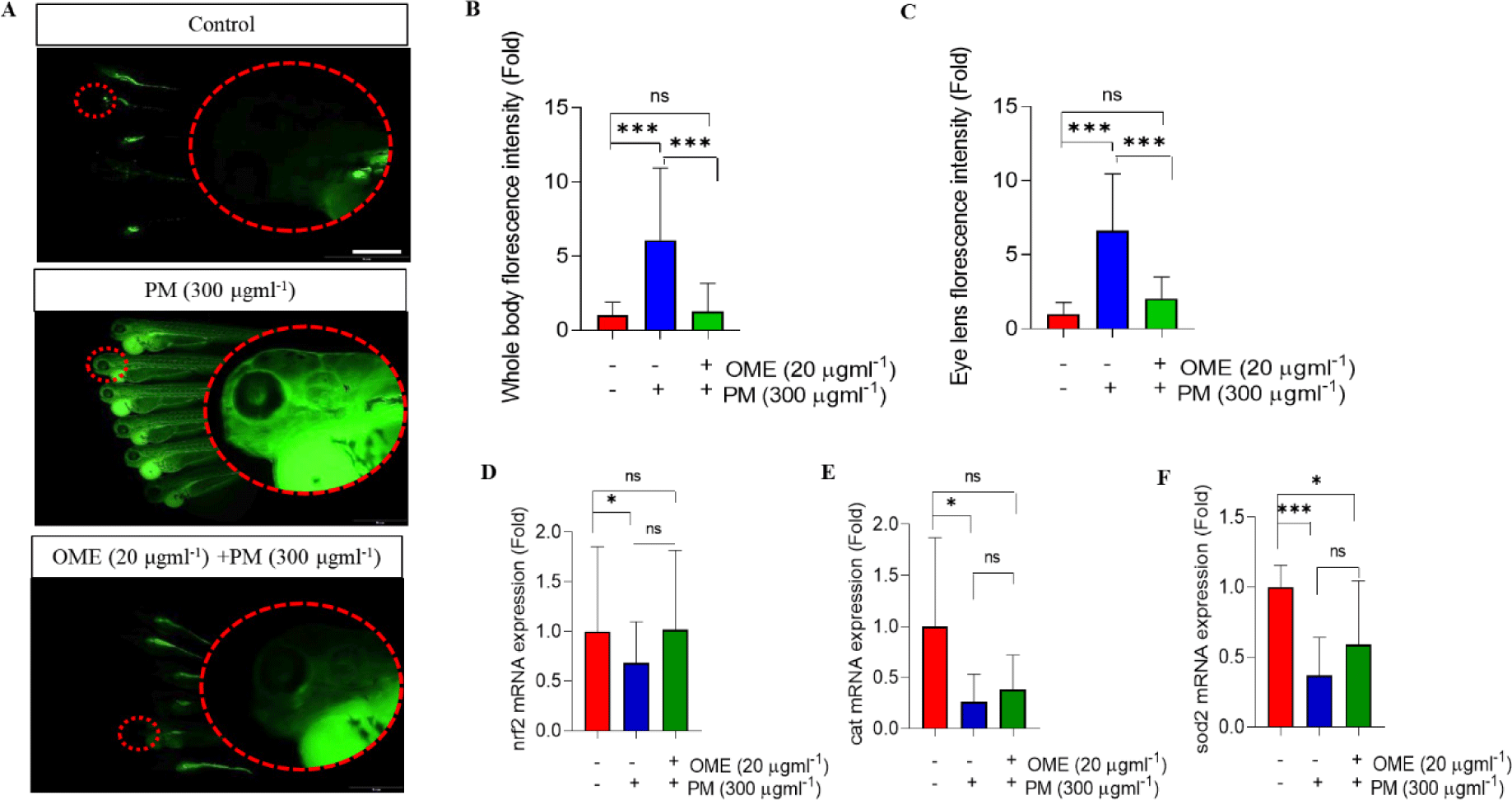
PM is known to induce oxidative stress (Valavanidis et al., 2013) and overproduction of ROS is a basal indicator of oxidative stress. So, we determined whether OME attenuates overproduction of ROS using 2',7'-Dichlorofluorescin diacetate (DCFH2-DA, Sigma-Aldrich) assay which is known to be a cell-permeable non-fluorescent probe which is de-esterified intracellularly and turns to highly fluorescent 2',7'-dichlorofluorescein (DCF) upon oxidation. In the present experiment, the ROS generated in whole body and eye of zebrafish embryos at 3 dpf was determined using DCFH2-DA at a concentration of 10 μM and incubated for 1 h at 28.5°C. The florescence images were taken using fluorescence microscope (SZX16). The embryos were anesthetized before taking a photo. Whole body and eye florescence intensity were measured in pixel using imageJ software.
GraphPad prism software version 8 (GraphPad software, Boston, MA, USA) was used in the analysis of the results obtained in the current study. One-way analysis of variance (ANOVA) was used to determine significant differences (*p < 0.05, **p < 0.01 and ***p < 0.001) among mean values of data from each experiment group. All experimental data were expressed as mean ± SD.
Results
A recent study reported that PM is toxic to not only humans but also zebrafish (Hyun et al., 2022; Ritz et al., 2019; Smoot et al., 2022). So, first we have confirmed whether the PM shown toxicity to the embryos. As expected, toxicity cause by PM exposure was observed as phenotype alterations such as shorten body length, cardiac and yolk sac edema, and blood accumulation, whereas all the phenotype alteration significantly reduced by OME pretreatment, and the reduction level was similar to that of the control (Fig. 1). Preservation of mortality, heartbeat, and malformation were similar to these results (Table 2). These suggest that OME pretreatment have protective effect against PM exposure.
Unlike other internal organs, the eyes are directly exposed to environmental pollution. Therefore, we thought that toxicity at a level that causes external deformities would have severe adverse effects on the eyes. So, we confirmed the effects on the eyes due to PM exposure. As the results, the size of eye area and lens decreased caused by PM exposure, whereas its reduction was preventive by OME pretreatment most similar to that of the control (Fig. 2A–2C). To clarify, we use Tg (mito:eGFP) transgenic zebrafish that expresses green fluorescence in all cells where mitochondria exist. Healthy cells expressed with fluorescence were observed to be clearly expressed in control and OME pre-treatment embryos, whereas the fluorescence intensity significantly decrease caused by PM exposure (Fig. 2D and 2E). This suggests that OME pretreatment attenuate eye morphology. Next, to determine whether these eye morphologic change in the eye also affect its function, the eye embryogenesis and development gene was examined. The mRNA expression level of both pax6a and pax6b were significantly downregulated in the PM exposure, whereas it was recovered by OME pretreatment compared to that of the control. In addition, the mRNA expression levels of crybb1 significantly downregulated caused by PM exposure while that of cryaa upregulated although not significant. OME pretreatment shown a trend of reducing these down- and up-regulation in a manner which was not significant compared to PM only group. Moreover, all the mRNA expressions levels of, vsx-1 and rho were significantly down-regulated in the PM exposure compared to that of the control. OME pretreatment up-regulated the mRNA expressions not significantly in atoh8, rho and significantly in vsx-1 compared to PM only group (Fig. 3). These results suggest that PM exposure caused eye malformation and OME prevent this adverse effect.
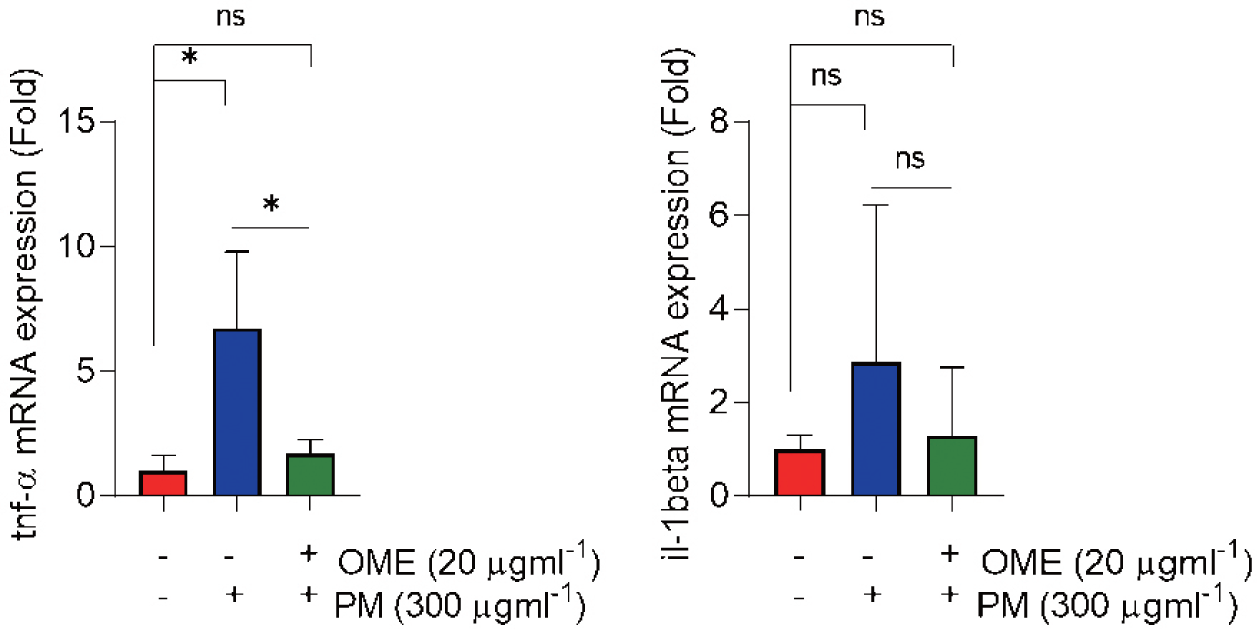
Oxidative stress and inflammation are hallmarks of toxicity, so, first, we determined whether OME protects against oxidative stress. H2DCF-DA assay was conducted at 72 hpf to determine ROS. Whole-body and eye ROS expression were significantly induced by PM exposure which was characterized by the increased relative fluorescence intensities in the whole-body and eye regions of PM-treated zebrafish embryos, whereas the overexpression was not observed by OME pre-treatment (Fig. 4A–4C). The mRNA expression level of antioxidant enzymes was determined. As the results show, nrf2, cat and sod2 mRNA expression levels were down-regulated in a PM exposure whereas the decreased nrf2, cat and sod2 expression level tended to increase by OME pre-treatment (Fig. 4D and 4F). Next, the mRNA expressions levels of pro-inflammatory cytokines that tnf-α and il-1β were up-regulated, whereas the both mRNA expression levels were down-regulated by OME pre-treatment (Fig. 5). These results suggest that suggesting that OME prevents oxidative stress and inflammation caused by PM.
Discussion
PM has a critical toxicological impacts not only humans but also animals (Losacco & Perillo, 2018).
A study has been shown that developmental toxicity increases, including increased mortality and pericardial edema, decreased hatchability, heartbeat on exposure to PM2.5 dose-dependent manners in zebrafish (Chen et al., 2023). The other study found that exposure to PM10 resulted in developmental abnormalities, including delayed yolk sac absorption, cardiovascular edema, shorten body length, decreased hatchability and heartbeat (Cen et al., 2020; Park et al., 2023). Both studies showed that multi-organ toxicity caused by PM exposure regardless PM size. The eyes, unlike other internal organs, directly affected by environmental pollutants such as PM. In this study, therefore, zebrafish embryo was used to establish an experimental in vivo model to explore preventive substances against PM exposure. The embryonic toxicology of PM in the present study was observed similar to those in scientific literature that significant reduction mortality and hatchability and increased malformations including increased yolk sac and cardiac edema were observed, whereas this toxicity was alleviated by OME pre-treatment. Moreover, it was confirmed that total eye and lens size also reduction caused by PM exposure and it was prevented by OME treatment.
The pathogenesis of microphthalmia caused by PM is poorly understood. However, PM exposure during pregnancy has been reported to become the leading cause for complications in fetal growth in early gestation period (Dejmek et al., 1999). Altogether, in vivo studies related to PM exposure damage to the eye area scarcity in scientific literature (Yang et al., 2019). In this study, there are significant incensements in both total eye and lens size reduction caused by PM exposure in zebrafish embryos. The significantly reduced mitochondria fluorescence in the lenses of zebrafish embryos gives clue about PM-induced possible damage to mitochondria. Chen et al. (2023) described the effect of PM to cause mitochondria dysfunction leading to ROS accumulation. In the study can be used as an initiative in possible therapeutic effect of OME pretreatment on PM-induced mitochondria damage.
The transcription factor pax6 (paired box 6) is a vital developmental regulator that plays a pivotal role in the development of the eye, brain, and olfactory system (Hart et al., 2013). Zebrafish has two pax6 genes, pax6a and pax6b, which are expressed in the developing eye (Delporte et al., 2008) and pax6 downregulation cause eye related diseases (Chen et al., 2013). In the present study, both pax6a and pax6b mRNA expressions levels were downregulated by PM exposure, but, OME pretreatment upregulated these mRNA expressions level in zebrafish embryos. On the other hand, crystallin genes, which predominant in lens cytoplasmic proteins, are crucial for establishing the lens’s gradient refractive index (Shiels & Hejtmancik, 2015). The α-crystallin, produced by epithelial and fiber cells, is expressed at the lens vesicle stage, while β- and γ-crystallin are unique to fiber cells, marking early fiber cell differentiation (Huang & Xie, 2010). In the study, PM exposure slightly upregulate α-crystallin (cryaa) mRNA expression level. Beta-crystallin B1 (crybb1), which encodes β lens protein, is expressed in the lens area of zebrafish eyes and is progressively increased during embryogenesis. Downregulation of crybb1 could potentially impact the lens development of zebrafish offspring (Chen et al., 2021). In this study, PM caused downregulation of crybb1 mRNA expression level. The differences in the cryaa and crybb1 expression time points and locations might have a role in PM induced differential relative mRNA expressions between the two genes.
Atoh8 (atonal basic helix-loop-helix [bHLH] transcription Factor 8) is important for the regulation of retina development during zebrafish embryogenesis (Yao et al., 2010). The homeobox gene vsx1 (visual system homeobox 1 homolog) is expressed in retinal in zebrafish embryos (Passini et al., 1998). The gene rho codes for rhodopsin belonging to the opsin plays a major role in hyperpolarization of rod and cones in the retina involved in the capture of the environmental light stimuli (Magnoli et al., 2012). All the three genes coding atoh8, vsx1 and rho are important in development and smooth function of retina in the zebrafish eye. In the present study, the mRNA expression levels of the genes significantly downregulated giving clues of possible retinal damage caused by PM. There are reports of PM exposure causing retinal damage basically due to incurred inflammation or other adverse effects like ferroptosis, endoplasmic reticulum stress in retina (Gu et al., 2022). But, interestingly, OME pretreatment upregulated the atoh8, vsx-1 and rho mRNA expressions levels compared to PM exposure group.
A central role is played by the ROS in adverse effects, particularly in the by PM exposure (Lee et al., 2020, 2021; Tien et al., 2020). In the present study found the protection effect by OME treatment against oxidative stress caused by PM induced ROS overproduction in eye of zebrafish embryos. PM caused oxidative stress was controlled by nrf2 (nuclear factor erythroid 2-related factor 2) pathway activation (Valavanidis et al., 2013). PM2.5 causing disruptions of nrf2 pathway and downregulating the antioxidant related proteins including catalase (cat), superoxide dismutase 2 (sod2) causing oxidative stress leading to inflammation (Cui et al., 2020). Interestingly, in this the study PM caused downregulation of nrf2, cat and sod2 mRNA expressions levels, as well as the mRNA expressions of pro-inflammatory cytokines that tnf-α (tumor necrosis factor alpha) and il-1β (interleukin 1 beta) were upregulated whereas OME pretreatment was recovered those mRNA expressions levels compared to PM exposure group. These results agree with the PM caused oxidative stress and inflammatory reactions (Cui et al., 2020; Valavanidis et al., 2013; Zhao et al., 2009).
In summary, PM exposure causes oxidative stress and inflammation, consequently causing eye development defect and these can be prevented by OME pretreatment.







