Introduction
The worldwide diabetes mellitus (DM) population has increased more than double during the past 20 years. Regarding the IDF report (2021), the number of DM patients among the adult population aged 20–79 is approximately 537 million, and the DM incidence rate is estimated to increase to 643 million in 2030 and 783 million in 2045. According to the World Health Organization (WHO), there was a 3% increase in diabetes mortality rates by age, and the number of deaths due to diabetes and diabetes with complication was estimated 2 million in 2019.
There are majority two types of DM are well known as type 1 (T1DM) and type 2 diabetes mellitus (T2DM; Roglic, 2016). T1DM is the selective autoimmune diseases causes by increased blood glucose levels due to the insulin deficiency that occurs as a result of the destruction of the pancreatic islet β-cells (Katsarou et al., 2017; Tehrani & Lin, 2011). T2DM a disorder of the characteristic regulation of carbohydrate, lipid, and protein metabolism due to problems with insulin secretion, insulin resistance, or a combination of the both (DeFronzo et al., 2015). Despite having different etiologies of these two types of DM, they fundamentally have a common cause, which is the inability to lower elevated glucose levels in the blood due to dysfunction to β-cells that secrete insulin.
It is well known that seaweed is rich in various bioactive compounds that improve health functions. There are epidemiological reports that consume group of seaweed have a lower incidence of chronic diseases such as hyperlipidemia and cardiovascular disease (Brown et al., 2014) and scientific evidence related to this is increasing. In addition, it has been reported that polyunsaturated fatty acids and phenolic compounds present in seaweed are effective in controlling metabolic diseases such as DM (Sharifuddin et al., 2015). Moreover, several studies have reported that seaweed has the ability to maintain cell function in cells such as β-cells (Cha et al., 2021), neuronal cells (Silva et al., 2018), and colon tissue cells (Martínez et al., 2021) amidst various diseases. Recently, it was reported that Polysiponia sp. derived construct inhibit the expression of inflammatory cytokines and skin barrier degradation in HaCaT keratinocytes (Jayasinghe et al., 2022) and anti-allergic effect was also reported in IgE/BSA-stimulated mast cell and passive cutaneous anaphylaxis mouse models (Kim et al., 2022). Interestingly, our previous study showed that Polysiphonia sp. crude extract improved insulin secretion by attenuating toxicity to pancreatic β-cells from free fatty acids and protecting β-cells (Cha et al., 2018), as well that Polysiphonia sp. derived construct improved insulin secretion function by inhibiting Parkin protein degradation in pancreatic β-cells (Cha et al., 2021). Like these, Polysiponia sp. has recently been reported to have excellent effects such as anti-inflammatory and improved insulin secretion function, but there are no reports on DM treatment and/or β-cell regeneration.
Zebrafish is the most widely used laboratory alternative animal these days. This fish has genetic homology similar to humans and is cost-effective and time-efficient, so it has becoming more popular as an alternative animal model for experiments. In particular, pancreatic β-cells function is similar to that of humans, so it is often used as a diabetes model (Cha et al., 2018, 2021; Zang et al., 2018).
Therefore, the current study, zebrafish was utilized as an an in vivo animal model system to determine whether Polysiponia sp. crude extract has a β-cell regenerative effect.
Materials and Methods
Adult zebrafish (Danio rerio) were kept in each 3 L tank with the following conditions: 28.5°C with a 12/10 h light/dark cycle. Zebrafish were fed twice a day with JAQNO color charasin (Hai feng, Nantou, Taiwan) food supplemented. Tg(-1.0ins:ntr:EGFP) transgenic zebrafish was obtained from the Fluorescent Reporter Zebrafish Cooperation Center (FRZCC 1072) of Korea University. The Tg(-1.0ins:ntr:EGFP) was generated with the fluorescent protein green fused to nitroreductase (NTR). Adult zebrafish were maintained and managed in accordance with the guidelines of the animal experiment ethics committee of Hanseo University.
P. morrowii was collected from Jeju coast in the Korea in January 2020 and taxonomy classification, nomenclature was identified by Prof. G. H. Kim (Gongju National University, Korea). P. morrowii was cleaned with tap water and freeze-dried. After that it was ground to make solvent extract. Briefly, the ground alga was extracted with ten volumes of 80% (v/v) methanol (in water, 80% MeOH) at room for 24 h. The extracted solution was evaporated under vacuum. After completely removing methanol, it was dissolved in dimethyl sulfoxide (DMSO, Duchefa Biochemie, Netherlands) and used in the experiment.
The fish, Tg(-1.0ins:ntr:EGFP) have used metronidazole (MNZ; Sigma, St. Louis, MO, USA) as a substrate for NTR-mediated cell ablation. So, in order to ablate β-cells, the embryos were treated with 5 mM MNZ (Curado et al., 2007) in egg water supplemented with 0.2 mM 1-phenyl-2-thiourea (PTU, Sigma, St. Louis, MO, USA; Khaliq et al., 2018). The MNZ treated at 3 to 4 days post fertilization (dpf) in dark and then the embryos (n=10–13) were treated with the PME from 4 to 6 dpf (Fig. 1). At 6 dpf, the embryos were observed phase and fluorescence image using SZX16 fluorescence microscope (Olympus, Tokyo, Japan). The β-cell fluorescence was quantified in zebrafish embryos after carefully selecting the florescent area by using ImageJ 1.53t software (National Institutes of Health, Bethesda, MD, USA).
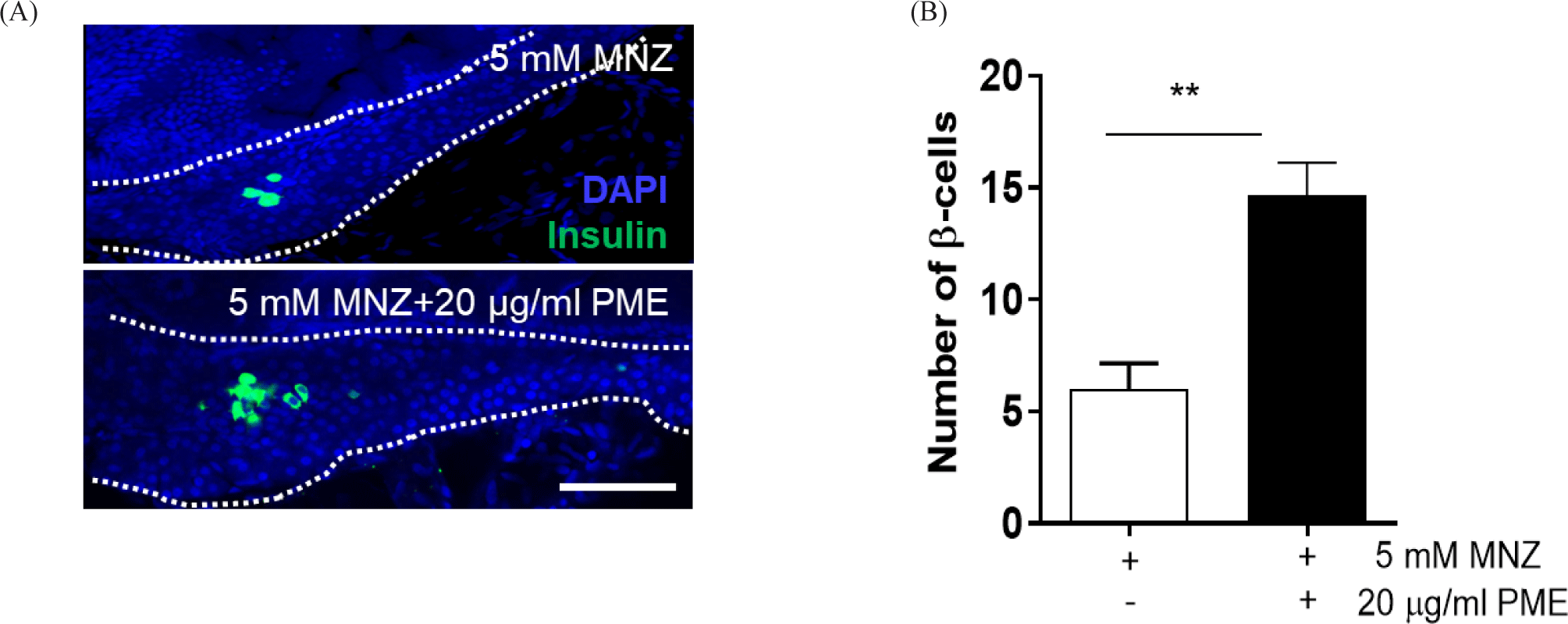
4% paraformaldehyde (PFA) used to fix the embryos for overnight at 4°C and washed with phosphate buffered saline (PBS) for 5 min at room temperature. After washing several times with PBS, the whole pancreas was isolated from the embryos, and subsequently mounted with glycerol-based mounting solution (Vector Laboratories, Newark, CA, USA), and observed using a confocal microscope (Nanoscope system, Daejeon, Korea). Number of β-cells were counted and quantified using ImageJ software.
All experiments were conducted in three different batches and the average value was calculated and presented. The Significance was tested using the Tukey test to analyse differences. All experimental data were expressed as mean ± SD. Among mean values of data from each experiment group, values of p < 0.05 considered significant.
Results
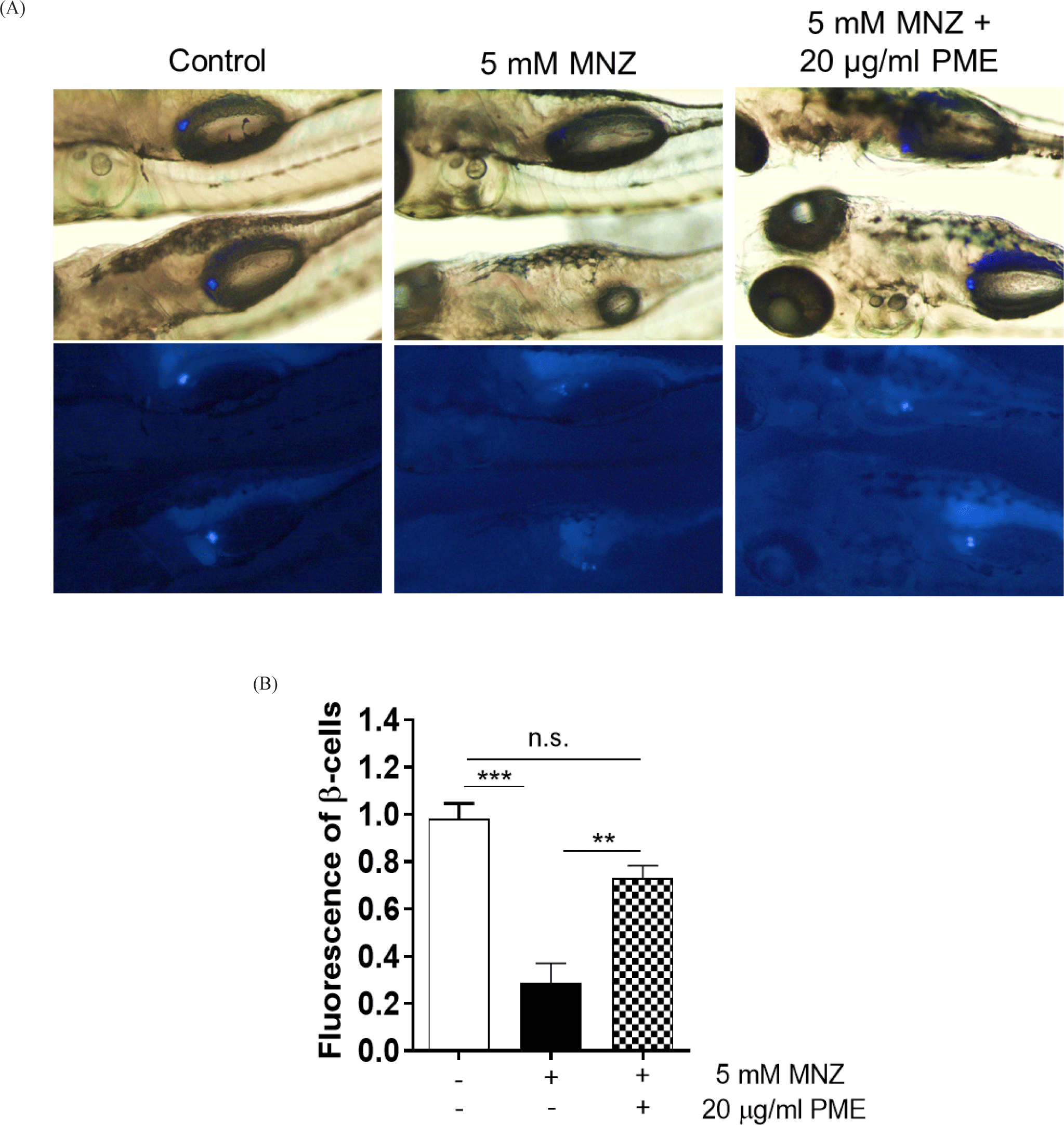
In order to ablate β-cell, the embryos were incubated with 5 mM MNZ for 24 h and the media was change with or without 20 µg/mL PME. And 48 h later, islet fluorescence of all embryos was reoccurred, larger islet was observed in PME treated embryos than MNZ only treated embryos (Fig. 2A), approximately 80% of islet were damaged by MNZ treatment, and this damage was recovered by approximately 50% by PME treatment. The degree of recovery was not significant compared to the control group, with islets of similar size as the control group (Fig. 2B). To clarify this, the pancreas was isolated from the embryo and observed the numbers of β-cells. As shown in Fig. 3, around six β-cells recorded in MNZ treated embryo, whereas around 15 β-cells recorded in the PME treated embryos. These results suggest that PME might be the potential treatment of the diabetes.
Discussion
Among the two major types of diabetes mellitus (DM), the cause of type 1 DM an autoimmune disease caused by genetic factor, which causes the self-destruction of β-cells (Katsarou et al., 2017). The main causes of type 2 DM are obesity, high blood sugar, and senescence, and secondary causes are stress and genetic factors, which lead to decreased β-cells function and destruction (DeFronzo et al., 2015). The common cause of these two types of DM is the destruction and dysfunction of pancreatic β-cells, which reduces insulin secretion and consequently causes DM.
Regardless of the type, treatment of DM patients requires a combination of medication and/or exercise, and diet to control blood sugar levels. Since most patients with T2DM are obese, they are prescribed exercise to reduce weight by restricting their diet. In cases where it is difficult to lower blood sugar levels through exercise and diet control, pharmacological treatment is prescribed (Khosla et al., 1995). However, almost DM drugs currently in use are treated through blood sugar control, and have many side effects such as headaches, dizziness, heart complications, liver damage, etc. (Chaudhury et al., 2017). Antidiabetic pharmaceutical prescribed as medications have many limitations in both efficacy and safety aspects, and as the number of DM patients are increasing worldwide, so that the demand for more efficient and safer diabetes pharmaceutical is increasing. Therefore, the current study, we have investigated a phytotherapy with PME a potential future drug to accomplish this goal. So far, there is no drug that treats DM by promoting the regeneration of β-cells. In addition, the use of animal models for the search for new drugs that promote β-cell regeneration takes considerable time and cost as well animal ethics. Therefore, we utilized a time-efficient and cost-effective in vivo model to search for new materials that promote β-cell regeneration. We found that such PME treatment directly accelerates pancreatic islet β-cell regeneration (Figs. 2 and 3).
It was interesting to find out that PME contributes to the speeding up of regeneration of pancreatic β-cells in the zebrafish. A zebrafish model of conditional β-cell ablation could be used to further support observations of β-cell proliferation accounting for β-cell mass expansion in zebrafish (Moro et al., 2009). The present study has shown an increase in β-cell mass when treated with 20 µg/mL PME compared to that of the control group. This provided evidence of the positive impact of PME on β-cell proliferation in the pancreas of zebrafish. A clear increase in florescence of insulin secretion in 20 µg/mL PME treated embryo when compared with their controls confirmed the PME treatment in β-cell proliferation. Importantly, the most critical function of β-cells is insulin secretion. The principle of fluorescence expression in the experimental model we used is that secreted insulin is expressed as green fluorescence. Therefore, the result performed that the regeneration rate was significantly increased by the PME treatment, the regeneration was performed that the function of the β-cells was also restored. Unlike other research, our results confirmed that seaweed, PME directly promotes pancreatic islet β-cell regeneration. This result has uncovered the role of PME in β-cells proliferation. So, this provides new light of information for the therapeutics of diabetics using seaweed. A new marine natural product like PME treatment in β-cell proliferation, regeneration and thus insulin secretion in zebrafish model could be the novel cure for human DM in the near future.
