Introduction
Toxic substances are a growing concern for environmental pollution throughout the world. Human activities such as mining, melting operations, metal and chemical industries, agriculture, and household activities are the principal sources of heavy metal contamination in the environment (Suciu et al., 2008). Due to their nature, toxicity, and propensity to accumulate in organisms, heavy metals are one of the most harmful categories among the toxic substances (Mortuza & Al-Misned, 2015; Tawari-Fufeyin & Egborge, 1998). Heavy metals accumulate in the living body and affect its normal functions (Budambula & Mwachiro, 2006).
Metals that enter the ecosystem may be taken up by organisms through bioaccumulation via the food chain and eventually become poisonous when the degree of accumulation rises significantly (Huang, 2003). Fish are often at the top of the aquatic food chain, making them more susceptible to accumulating trace metals in their tissues in contaminated environments (Mansour & Sidky, 2002). Fish health depends on proper concentrations of heavy metals, which are crucial parts of fish metabolism. Fish are commonly recognized as a prominent bioindicator of aquatic ecosystems due to their high trophic level and role as a substantial supply of balanced protein in the human diet (Rahman et al., 2012).
The river Padma, one of the major river of Indian subcontinents runs through the Rajshahi division. It is the main Ganges tributary, flowing 120 kilometers (km) generally southeast to its confluence with the Meghna River close to Goalandaghat. From its point of entry at Manakosa and Durlabpur unions in Shibganj upazila of Chapai Nawabganj district, the Ganges is commonly referred to as the Padma in Bangladesh.
The freshwater fisheries are a major component of Bangladeshi economy, representing a valuable source of income. Among the commercially important and available freshwater fish species, Punti (Puntius ticto), Peoli (Aspidoparia jaya), Bacha (Eutropiichthys vacha), Tengara (Mystus cavasius), and Hilsa (Tenulosa ilisha) have a significant socioeconomic impact on Bangladesh. To secure the supply of healthy fish, it is crucial in the aforementioned context to not only determine the extent of the harm but also to create a plan of action to address the issue of trace metal toxicity in Bangladeshi. This study aims to assess the concentration of select toxic heavy metal (Co, Zn, Mn, Cu, Cd, Cr, Pb, and Ni) concentrations in the water, sediments, and in the aforementioned fish tissues (skin, gills, and muscle) collected from the Padma River, Bangladesh. To determine the risk to human health for fish consumers, the level of heavy metal contamination in fish tissues was compared to globally accepted limits.
Materials and Methods
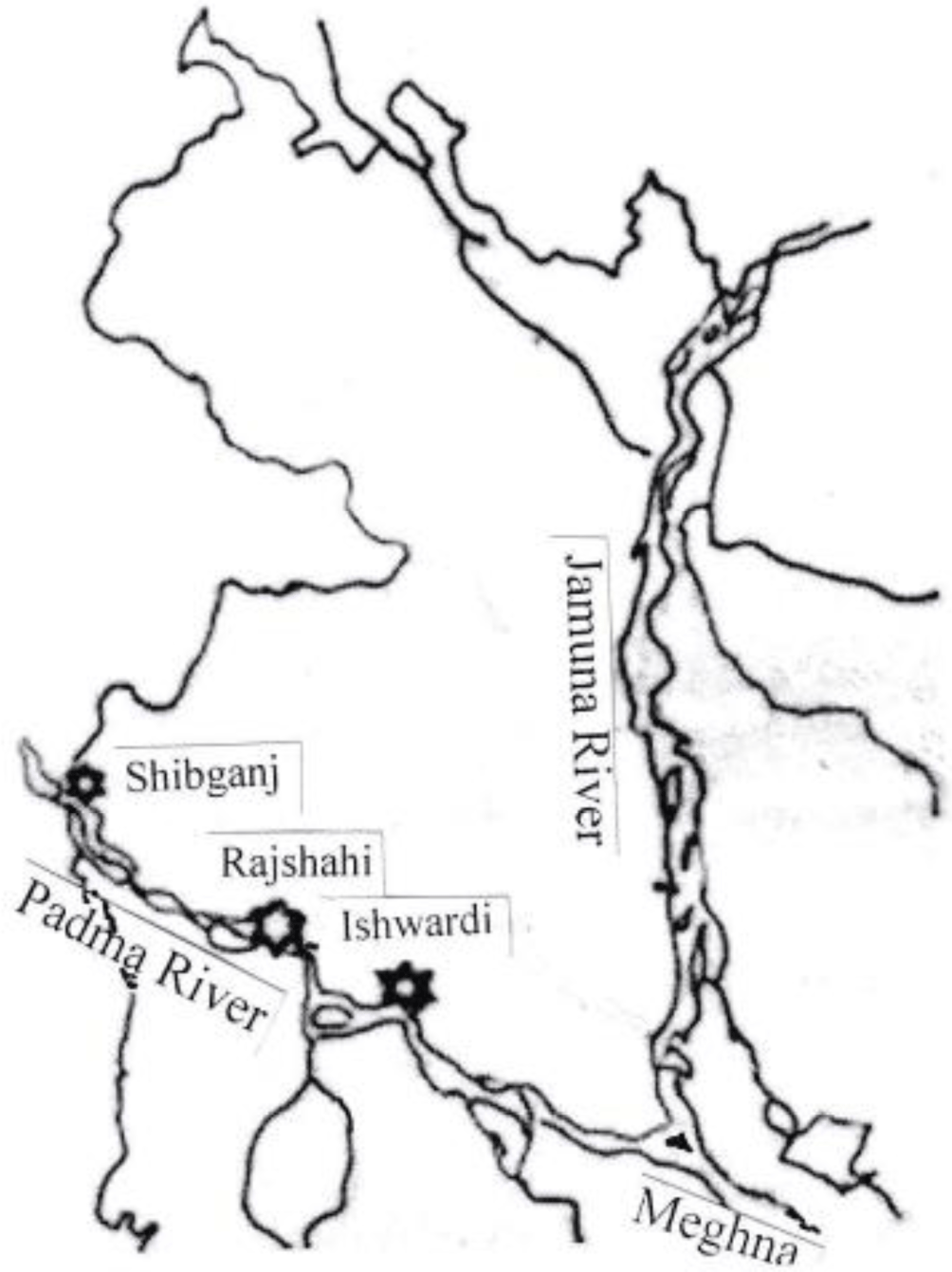
The present study focused on the Padma River from Pakshi, Ishwardi of Pabna District between 24°648621' north latitudes and 88°039439' east longitudes extending to Shibganj upazila of Chapai Nawabganj District between 24°068904' north latitudes and 89°033010' east longitudes to examine heavy metal contamination sources that affect the study area. There were three sampling spots, namely, Pakshi, Ishwardi, Pabna (spot 1), the Padma River near Rajshahi City (spot 2), and Shibganj of Chapainawabganj (spot 3). The sampling spots have urban, agricultural, and industrial amenities that were taken into consideration for this study (Fig. 1).
Samples (fish, water, and surface sediment) were collected from the sampling spots (Ishwardi, Pabna, Rajshahi City, Rajshahi, and Shibganj, Chapainawabganj) of Padma River; Each of the spots were around 70–100 km away from each other (Plate 1). Water samples were collected in pre-cleaned plastic bottles following filtration through micro-filter (No. 42 filter paper, Little Chalfont, Whatman, Maidstone, UK) and kept in refrigeration until analysis.
Surface sediment samples were taken using a stainless-steel grab sampler that allows free water to pass through the sampler during downward penetration. The water and surface sediment were collected from the same sampling spot. The sediment samples were first allowed to dry for a few days in the air atop Pyrex petri dishes before being baked in a laboratory oven at 105°C.
At each sampling time, five fish of each species (Punti, Peoli, Bacha, Tengara, and Hilsa) were collected from the Padma River or fish markets near the same sampling spots (plate 2). The fish were euthanized using percussion–based stunning. Then, they were put in a cooler filled with ice and transported to the lab. The tissue were dissected using a special ceramic knife, scissors, and plastic forceps to avoid metal contamination (Miyako, Osaka, Japan). Following a double-distilled water (DDW) wash, tissues were placed in Petri dishes and dried at 100°C until they reached a constant weight.
The dried fish tissues were digested according to the method of Hanson as described by Rahman et al. (2012). Briefly, 0.5 g of dried fish muscle, gill, and skin tissues were placed in a digesting device together with 2.5 mL of concentrated H2SO4 and 4 mL of concentrated HNO3. Using a hotplate, the mixture was gradually warmed to 60°C for 20 minutes and allowed to cool to room temperature (Rahman, 2004).
For each sample, three replicas were prepared for digestion. A teflon beaker was used to transfer samples, and DDW was used to bring the volume to 100 mL. A PTFE syringe filter (pore size, 0.45 μm) was used to filter the mixture and then placed in a screw-cap plastic tube. The data were represented using the average results from three independent analyses of each sample.
Sample analysis was performed at the Central Science Laboratory, University of Rajshahi. The detection of heavy metals (Zn, Mn, Cu, Cd, Cr, Pb, and Ni) in all samples (water, sediments, and tissues) was carried out using a flame atomic absorption spectrophotometer (AAS). Standard solutions made in the same acid matrix were used to calculate concentrations for analytical blanks that were run in the same manner as the samples. On the basis of the mono-element certified reference solution AAS Standard (Merck, Darmstadt, Germany), standards for the instrument calibration were created. After soaking in a 10% nitric acid solution overnight, all laboratory plastics and glassware were rinsed with deionized water.
According to the following Equation (1) published by Bortey-Sam et al. (2015), the estimated daily intake (EDI) was calculated.
Where MC is the average amount of heavy metals found in fish muscle tissue (in g/g), FDC is the average amount of fish consumed daily (in g/person/day), which is 49.5 g/person/day in Bangladesh (BBS, 2011), and BW is the body weight (which for adults is typically 70 kg). A g/kg bw/day was used to represent EDI.
THQ are used to calculate non-cancer risk estimates and to evaluate the potential health effects of heavy metal exposure. The USEPA region 111 danger-based concentration table was used to calculate the target hazard quotient in order to assess the danger to human health from consuming metal-contaminated fish (USEPA, 2011). The following Equation (2) was used:
Here, EF means exposure frequency (365 days/year), ED indicates the exposure duration (70 years) for non-cancer risk as used by USEPA, FDC is the daily food consumption of fish (49.5 g/kg d.w.) (BBS, 2011). MC indicates heavy metal concentration in tissue (μg/kg w.w.), CF denotes the conversion factor 0.208 (to convert fresh weight to dry weight by considering 79% of moisture content), and RfD is the reference dose of individual metal (μg/g day; Zn = 0.3, Mn = 0.14, Cu = 0.04, Cd = 0.001, Cr = 0.003, Pb = 0.004, Ni = 0.02) (USEPA, 2015), BW is the average adult body weight (70 kg) and ATn is the average exposure time for non-carcinogens (10,950 days) (USEPA, 2011). If the THQ value is less than 1, it is of less concern. Conversely, a potential health risk exists if the THQ is equal to or greater than 1 (Wang et al., 2005). Table 1 shows the parameters and values used in THQ estimation.
| Sampling spot | Amount of heavy metal (ug/L) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | Surface sediments | |||||||||||||
| Zn | Mn | Cu | Cd | Cr | Pb | Ni | Zn | Mn | Cu | Cd | Cr | Pb | Ni | |
| Shibganj, Chapai Nawabganj | 7.88±1.57 | 10.71±1.56 | 5.10±2.94 | 1.75±1.07 | 5.85±1.84 | 5.38±1.02 | 3.21±0.96 | 12.75±2.99 | 21.28±3.96 | 8.02±0.85 | 1.89±0.07 | 6.29±1.51 | 5.13±0.62 | 2.16±1.04 |
| Rajshahi City, Rajshahi | 10.29±1.62 | 11.08±1.75 | 6.32±1.86 | 1.58±1.14 | 6.11±4.05 | 4.51±2.18 | 2.48±1.82 | 20.10±4.20 | 23.31±4.98 | 9.64±1.57 | 2.84±0.52 | 5.56±0.64 | 4.84±0.59 | 3.52±0.31 |
| Irshardi, Pabna | 8.69±1.31 | 10.52±1.43 | 6.21±1.39 | 0.87±0.56 | 4.75±3.87 | 4.45±1.62 | 2.81±0.08 | 18.29±3.85 | 20.72±4.65 | 6.52±1.83 | 1.28±0.86 | 4.74±1.19 | 4.79±0.98 | 2.48±0.43 |
| Mean ± SD | 8.95± 1.50 | 10.77±1.58 | 6.88±2.06 | 1.40±0.90 | 5.57±3.25 | 4.11±1.61 | 2.83±0.95 | 17.04±3.68 | 21.77±4.53 | 8.06±1.17 | 2.00±0.48 | 5.53±1.11 | 4.25±0.73 | 2.72±0.59 |
| WHO (2011) | 5,000 | 50 | 2,000 | 3 | 50 | 10 | 70 | 123 | NV | 25 | 6 | 25 | NV | 20 |
| USEPA (2012) | 5,000 | NV | 1,300 | 5 | 100 | 15 | NV | 110 | 30 | 25 | 6 | 25 | 40 | 16 |
The THQ of each metal is added together to create the “hazard index”, which is used to evaluate the combined potential harm caused by various metals (USEPA, 2011). It was assumed that no chronic danger was expected to exist at the site where the carcinogenic HI did not surpass the value of 1. If the hazard index is greater than one as a result of adding multiple HI, it would be appropriate to separate the compound by outcome and mechanism of action and derive HI for human health.
The CR estimation method uses a carcinogenic slop factor for those metals that, upon exposure, have a risk of developing cancer. For Pb, Cd, Cr, Cu, and Ni, carcinogenic slop factor values are available (Pb = 0.0085, Cd = 0.38, Cr = 0.5, Cu = 1.7, Ni = 0.9 [Agency for Toxic Substances and Disease Registry, ATSDR]). The appropriate number for lifetime cancer risk is between 10–4 and 10–6. The following Equation (4) can be used to estimate CR (USEPA, 2011):
Whereas AT denotes average time, carcinogens (365 days per year for 70 years), and CSF stands for oral slope factor of specific pollutant (mg/kg/day) (USEPA, 2000).
The following Equation (5) was used to determine the CRt of locals brought on by lifetime exposure to probable carcinogens (USEPA, 2011).
The cumulative cancer risks from all the carcinogens are added together if there are many (assuming cumulative effects). According to Cao et al. (2015), risks between 1.0 × 10−6 and 1.0 × 10–4 are acceptable.
Results
Heavy metal concentrations in water and soil sediment in different spots of the Padma are presented in Table 1. Heavy metal concentrations in the water of the Padma were found to be in the order of Pb > Cr > Cu > Mn > Zn > Cd > Ni at Shibganj of Chapai Nawabganj, in the order of Pb > Cr > Cu > Zn > Mn > Ni > Cd near Rajshahi City, and in the order of Pb > Cr > Cu > Mn > Zn > Cd > Ni at Irshardi, Pabna.
Similarly, heavy metal concentration in soil was found to be in the order of Pb > Cu > Cr > Mn > Zn > Ni > Cd at Shibganj of Chapai Nawabganj, in the order of Pb > Cr > Mn > Cu > Zn > Ni > Cd in Rajshahi City, and in the order of Pb > Cr > Zn > Cu > Mn > Cd > Ni at Ishwardi, Pabna. The overall average orders of metals in the water and soil samples of three spots were as follows: Pb > Cr > Cu > Mn > Zn > Cd > Ni (Table 1).
The mean concentration of metals determined in the water samples ranged from 5.24–26.26 µg/L and for sediments, the range was 10.13–38.21 mg/kg. The metals determined were Zn, Mn, Cu, Cd, Cr, Pb, and Ni, with mean concentrations of 9.65, 9.68, 13.33, 5.69, 15.94, 26.26, 5.24 (mg/L) in water and 21.30, 24.46, 28.26, 10.73, 32.72, 38.21, 10.13 (mg/kg) in sediment, respectively. The concentration of Pb was highest in water and sediment, and the concentration of Ni was lowest for both cases.
The average heavy metal contents in different fish tissues (muscle, skin, and gills) are presented in Table 2. The accumulation patterns of all the metals were more or less similar among the species, organs, and sampling sites. When fish are exposed to elevated levels of metals, they can absorb and then bioaccumulation these metals through their gills and skin or by ingesting contaminated water and food. The highest concentration of Mn was detected in T. ilisha (34.15 mg/kg) in the gills, and the lowest concentration of Cd was found in the muscle of P. ticto (0.32 mg/kg). A comparatively low Cd concentration was found in all fish tissues (Table 2). The average heavy metal concentration in different organs of the fish are in the order of: Mn > Zn > Pb > Cu > Cr > Pb > Ni > Cd.
Through the Pearson correlation matrix calculation among the heavy metals, the sources of heavy metals can be detected (Table 3). The relationship between heavy metals may offer remarkable information on the sources and pathways of heavy metals. Correlation analysis shows significant positive correlation between Zn-Mn (r = 0.908), Zn-Cd (r = 0.887), Mn-Cd (r = 0.823), Cu-Cd (r = 0.878), and Zn-Ni (r = 0.839) at p < 0.01 level, whereas significant correlation exists between Zn-Cu (r = 0.687), Mn-Ni (r = 0.712), Cd-Ni (r = 0.792), and Cr-Ni (r = 0.796) at p < 0.05 level, lastly Cu-Cr (r = 0.72), Mn-Pb (r = 0.650), and Cd-Pb (r = 0.537) are significantly but inversely correlated with each other at p < 0.05 level (Table 3).
Based on the assumption that each person weighs 70 kg and has had an exposure time of 70 years, the EDIs for the seven heavy metals under study are calculated. Table 4 shows that the maximum daily intake is in the following order: Zn > Mn > Cu > Cr > Pb > Ni > Co = Cd, while the estimated findings are much lower than the normal TDI established by the various international organizations (Table 4).
| Heavy metal | EDI (µg/kg b.w./day) | TDI (µg/kg b.w./day) | Reference | ||||
|---|---|---|---|---|---|---|---|
| Puntius ticto | Aspidoparia jaya | Eutropiichthys vacha | Mystus cavasius | Tenulosa ilisha | |||
| Zn | 9.864 | 8.528 | 8.308 | 10.218 | 13.585 | 430 | SCF, 2003 |
| Mn | 16.016 | 11.186 | 8.839 | 14.6856 | 19.042 | 50 | WHO, 2010 |
| Cu | 0.940 | 1.202 | 0.976 | 1.216 | 1.365 | 60 | USEPA, 2015 |
| Cd | 0.354 | 0.368 | 0.297 | 0.453 | 0.509 | 1 | WHO, 2010 |
| Cr | 1.796 | 1.075 | 1.789 | 2.001 | 1.838 | 30 | EFSA, 2014 |
| Pb | 1.718 | 1.853 | 1.741 | 1.874 | 1.896 | 2 | WHO, 2010 |
| Ni | 1.534 | 0.955 | 1.534 | 2.206 | 2.489 | 20 | EFSA, 2015 |
The study showed that THQ and HI values in fish and individual metals were less than 1, indicating no potential risk regarding the studied heavy metals. The THQ of the heavy metals decreased in the following order: Cr > Pb > Cd > Mn > Ni > Zn > Cu. When THQ and HI values are less than 1, there is no obvious risk to the population, but if these values exceed 1, there may be concern for potential non-carcinogenic effects (USEPA, 2004). For the adult population, calculated values of THQ were less than one in the food intake pathway. Calculated values for the HI for adults who consume these fish species were less than one (1). HI values were calculated as 0.3389, 0.2861, 0.4026, 0.3885, and 0.4100 for Punti, Peoli, Bacha, Tengara, and Hilsa, respectively (Table 5). HI was less than 1, indicating that the adult population was not at risk from non-carcinogenic effects. Results indicate that five fish species were found to be safe for human consumption.
CR was only calculated for Cu, Cd, Cr, Pb, and Ni due to the availability of the carcinogenic potency slope factor of the carcinogens for those metals (Table 6) (Liu et al., 2018). CR values of Cu, Cd, Cr, Pb, and Ni ranged from 4.00 × 10–8 to 6.35 × 10–6 (Table 7), all less than 10–4, and CRt values ranged from 9.85 × 10–6 to 1.10 × 10–5, indicating no potential CR from fish consumption in the Padma River. Although fish under the present findings are safe for human consumption, the probability of contracting cancer is still present for continuous consumption for 70 years or more in the future.
| Elements | Classification by IARC1) | RfD (ug/g.d) | Source | CSF (ug/g.d)–1 | Source |
|---|---|---|---|---|---|
| Zn | 3.00 × 10–1 | a/EPA | - | - | |
| Mn | - | 1.40 × 10–1 | a/IRIS | - | - |
| Cu | 4.00 × 10–2 | a | 1.70 × 10–1 | - | |
| Cd | 1 | 1.00 × 10–3 | IRIS2) | 3.80 × 10–1 | CALEPA3)/USDOE, 2011 |
| Cr | 1 | 3.00 × 10–3 | 19,24 | 5.00 × 10–1 | CALEPA3)/19, 24 |
| Pb | 2B | 4.00 × 10–3 | WHO4) | 8.50 × 10–1 | CALEPA3) 37 |
| Ni | 1 | 2.00 × 10–2 | IRIS | 9.10 × 10–1 | CAFEPA3) |
Discussion
Heavy metal contamination in water, surface sediments, and fish can increase human health risks through various exposure routes. In the present work, non-carcinogenic and carcinogenic health risks caused by fish consumption were explored.
The agricultural field runoff during the rainy season and the disposal of domestic wastes at various points along the length of the river, which are known to contain heavy metals like Zn, Mn, Cd, Cr, Cu, Pb, and Ni eventually end up in this aquatic ecosystem, which could be leading to heavy metal pollution in the area (Woodworth & Pascoe, 1982). Mn has the highest concentration of any of these elements, with average concentrations of 10.77 mg/L and 21.77 mg/kg in sediment and water, respectively. Ni, on the other hand, has the lowest concentrations, with levels in water and sediment of 1.40 mg/L and 2.00 mg/kg, respectively. These findings concur with those published by Opaluwa et al. (2012).
The skins of fish are studied in the context of heavy metal contamination due to its contact with the contaminated medium (water) and also having the thinnest epithelium of any organ (Bebianno et al., 2004). The concentration of metals in the water is reflected in the metal levels in the gills, whilst the concentration in the muscle is often related to the long term storage of pollutants in the fish’s body (Romeo et al., 1999). The muscle, on the other hand, might be a reliable sign of long-term exposure (Bellinger, 1992). For some of the metals in this study, higher buildup was seen in the gills. Heavy metal accumulation in fish may be influenced by both exogenous and endogenous variables, which could explain the variation in the results. According to Jovanović et al. (2017), metals build up in bottom feeders and biomagnify their way up the food chain.
The investigation of heavy metal correlations can be used to determine the origin and migration of metals (Rahman et al., 2011). Metals with a positive correlation may originate from the same sources (Üstün, 2009). These incredibly strong positive correlations amongst heavy metals point to the potential of shared anthropogenic origins (Armah et al., 2010). The remaining elemental pairs, on the other hand, do not significantly correlate with one another, indicating that these metals are not linked together. This could be indicative of point sources of heavy metal pollution.
The daily intake of the metal (EDI) was taken into consideration while calculating the total hazard quotient (THQ). The USEPA states that the acceptable THQ threshold value is 1. THQ levels below the unit limit imply that there would be no adverse effects on lifetime consumption from exposure to the contaminants (Yi et al. 2011). However, each of the metals analyzed had a THQ that was less than 1. Therefore, the individual THQ of the seven metals show no risk to human health. The research focused on the assessment of HI, in which exposures that exceeded HI units (1) highlights a critical issue of health concerns for local consumers (Liu et al., 2018). The examined HI did not exceed the recommended level, but the HI values followed the THQ pattern, indicating that consuming those fish species would not have any non-carcinogenic health effects on consumers.
The incremental chance that a person may get cancer over the course of their lifetime when exposed to a possible carcinogen is known as CR (Zhong et al., 2018). The tolerable limit for lifetime exposure to carcinogens was defined between 10–4 and 10–6 (risk of acquiring cancer over a lifetime is 1 in 10,000 to 1 in 1,000,000) (FAO, 2014; Yin et al., 2015). The likelihood that someone may acquire cancer is greater than 1 in 100,000 when CR levels are higher than 10–5 (Traina et al., 2019). Due to the limited availability of the cancer slope factor (CSF) (Table 6), the CR was only computed for Cu, Cd, Cr, Pb, and Ni (Liu et al., 2018). For the aforementioned metals, the projected CR values for all fish were less than 10–4 (Table 7). CR values under 10–6 signify a low CR exposure and CR values above 10–4 on the other hand, signify a strong CR exposure (Sultana et al., 2022; USEPA, 1989; USEPA, 2000). In this study, the CR values of Cu, Cr, and Ni of the five species of fish from the Padma River showed a possible CR, as level of contamination crossed 10−6 (Keshavarzi et al., 2018). Therefore, consuming the aforementioned five fish species from the Padma River over an extended period of time may have carcinogenic effects. To establish a more accurate risk assessment, attention should be given to the various exposure pathways, such as inhalation and cutaneous exposure.
Conclusion
Heavy metal concentrations in fish samples were found to be generally greater than those in water and surface sediments. The gills of the five fish species contained higher concentrations of heavy metals than the skin and muscle. From this study, it is revealed that the heavy metal concentrations in the water, surface sediments, and fish species were below the WHO, FAO, and EPA acceptable limits. The EDI value in muscle was also lower than the TDI for all five fish obtained from the Padma River. The THQ and HI were less than 1 for all detected heavy metals in the muscle of fish, suggesting no non-CR to consumers. CR values of Cu, Cd, Cr, Pb, and Ni ranged from 4.00 × 10–8 to 6.35 × 10–6, all less than 10–4, and CRt values ranged from 9.85 × 10–6 to 1.10 × 10–5, indicating some pose a CR from consumption of those fish from the Padma River. Attention should be focused on the other exposure pathways, such as inhalation and dermal exposure, to determine a more precise risk assessment.