Background
Germline stem cells are a very important cell type taking charge of gamete production (Brinster, 2007; Spradling et al., 2011; Lehmann, 2012). Due to their high application possibilities to animal transgenic research as a mediator conveying new traits to the next generation, lots of trials for in vitro culture and manipulation of them have been conducted in many mammalian (Guan et al., 2006; Aponte et al., 2008; Lee et al., 2013; Tiptanavattana et al., 2013; Lee et al., 2014) and some avian species (Park et al., 2008; Song et al., 2014). In fish, similar studies have been performed in small fish models (Sakai, 2002; Hong et al., 2004; Fan et al., 2008; Kawasaki et al., 2012; Wong and Collodi, 2013; Wong et al., 2013; Li et al., 2014), but the related ones dealing with large farmed fish have been rarely conducted (Shikina et al., 2008; Shikina and Yoshizaki, 2010; Lacerda et al., 2013). Therefore, establishment of an in vitro culture system for germline stem cells derived from the economically valuable fish is one of the upcoming challenges in the field of fish transgenic research. As one of the important aquaculture fish, the Siberian sturgeon (Acipenser baerii) provides valuable food resources including a luxury food and caviar and has a merit in culture due to its ability to tolerate the changes of environmental factors such as temperature and low O2 concentration (Gisbert and Ruban, 2003). Thus, to fulfill the improvement of the breed of this species, applying transgenic technology is a worthwhile work in regard to commercial aspect. Moreover, establishment of germline stem cells can contribute to species preservation of this endangered fish species (Ruban and Zhu, 2010; Hong et al., 2011; Lacerda et al., 2014).
To establish germline stem cell culture system in A. baerii, developing appropriate feeder cell lines that can support germline stem cells in vitro is a top priority. In general, cell lines derived from gonads are known to possess a capacity to support survival, maintenance, growth, and differentiation of germline stem cells during in vitro culture (Hofmann et al., 2003; Nagano et al., 2003; Hong et al., 2004). It has been reported that mouse spermatogonial stem cells formed colonies and maintained its characteristics for 5 months on testis-derived feeder cells during in vitro culture (Kanatsu-Shinohara et al., 2012). In fish, trout spermatocytes showed longer survival on Sertoli cells from testis than those without somatic cells in culture (Loir, 1989). Moreover, zebrafish ovarian-somatic feeder cells supported cell growth, survival, and germline competency of female germline stem cells in culture (Wong et al., 2013).
Therefore, in this study, we tried to establish A. baerii gonad-derived cell lines as a first step toward culturing A. baerii germline stem cells. Subsequently, we evaluated optimal growth conditions of the established cell lines and characterized them within the framework of the morphology, DNA contents, and expression of genes that are known to express in A. baerii gonad. In addition, considering the legal and ethical limitation about killing this endangered species repeatedly to secure a large amount of feeder cells, we tested the feasibility of long-term culture and cryopreservation of the established cell lines.
Methods
Siberian sturgeons (A. baerii) were reared by a water recycling system in hatchery of Pukyong National University (Busan, Korea) at ambient temperature and fed an artificial feed (Millennium Plus; Woosung Feed Corp., Daejeon, Korea). Five 1-year-old fish were used in this study, and the average body length and weight were 39.6 ± 3.3 cm and 192.3 ± 54.8 g, respectively. All procedures for animal management, euthanasia, and surgery were complied with the guidelines of Institutional Animal Care and Use Committee (IACUC) of Pukyong National University and the ethical guidelines published by International Council for Laboratory Animal Science (ICLAS).
Gonad tissues were dissected from the body with sterile scissors and tweezers following disinfection with 70 % ethanol (SK Chemicals, Sungnam, Korea). Each gonad was washed twice with Dulbecco’s Phosphate-Buffered Saline (DPBS; Gibco, Grand Island, NY, USA) containing 1 % (v/v) penicillin and streptomycin (P/S; Gibco) in petri dishes (SPL Life Sciences, Pocheon, Korea) and placed in a 35-mm petri dish (SPL Life Science) filled with digestive solution consisting of a Leibovitz’s L-15 Medium (L15; Gibco) supplemented with 500 U/mL collagenase type I (Worthington Biochemical Corporation, Lakewood Township, NJ, USA) and 0.05 % trypsin EDTA (Gibco). Then, the tissues were chopped using a surgical blade and incubated for 30 min at 28 °C. After digestion, enzyme was inactivated by adding 10 % (v/v) fetal bovine serum (FBS; Gibco)-containing L15. All the tissue derivatives were filtered on 40-μm cell strainers (BDFalcon™, San Jose, CA, USA), and the isolated cells were retrieved by centrifugation at 400×g for 4 min. Viable cells were counted with a hemocytometer (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany) after trypan blue (Gibco) staining, and 5 × 105 live cells were seeded in 24-well culture plates (SPL Life Sciences) with L15 containing 20 % (v/v) FBS and 1 % (v/v) P/S. The cells were cultured at 28 °C with an air atmosphere, and the culture media were changed every 2 to 3 days. Subcultures were conducted when the cells reached 90 to 100 % confluency. For subculture, the cells were washed twice using DPBS supplemented with 1 % (v/v) P/S and detached by 0.05 % trypsin EDTA at room temperature for 5 min. After trypsin inactivation by adding one volume of 10 % (v/v) FBS-containing L15, the detached cell suspension was centrifuged at 400×g for 4 min. Then, the harvested cells were resuspended with culture media and subcultured at a 1:3 ratio up to the 7th passage and since then at a 1:5 ratio.
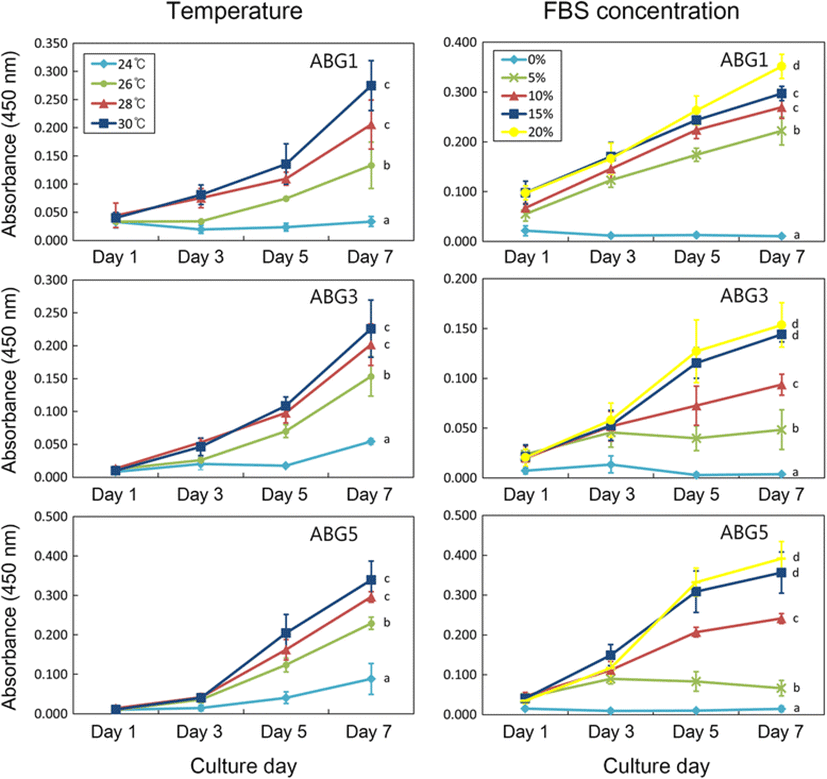
To investigate the growth rates under different culture conditions, 2.5 × 103 cells from each of three cell lines (designated as ABG1, ABG3, and ABG5) at passages 24 to 29 were seeded in 96-well microplates (Thermo Scientific, Vernon Hills, IL, USA) filled with L15 containing 20 % (v/v) FBS and 1 % (v/v) P/S and cultured under four different temperatures of 24, 26, 28, and 30 °C. Cell viability of each group was measured at days 1, 3, 5, and 7 after cell seeding using Cell Counting Kit-8 (CCK-8; Dojindo, Kushu, Japan) according to the manufacturer’s instructions. After determination of optimal culture temperature, a test for determining optimal FBS concentration for cell growth was conducted under the optimal culture temperature. The same protocols with temperature test were used, but the cells were cultured in five different media consisting of L15 containing different FBS concentrations of 0, 5, 10, 15, and 20 % under a fixed culture temperature. This experiment was conducted in triplicate.
From each cell line, 1 × 107 cells at passages 54 to 55 were harvested by trypsinization and centrifugation. Cell pellets were suspended in 1 mL DPBS at room temperature and were transferred into 4 mL absolute ethanol at −20 °C. After overnight at −20 °C, the cells were harvested by centrifugation and rehydrated in 5 mL DPBS for 15 min at room temperature. After that, RNase A (Bioneer, Daejeon, Korea) was treated with a final concentration of 200 μg/mL in DPBS for 30 min at room temperature and, subsequently, stained by propidium iodide (Invitrogen, Carlsbad, CA, USA) with a final concentration of 10 μg/mL in the dark for 1 h at room temperature. Finally, DNA content was measured by an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA). A previously established A. baerii heart-derived cell line, the ploidy of which was confirmed (Kim et al., 2014), was used as a control of normal diploidy.
Total RNA was extracted from each cell line using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) at passages 15 to 16 and passages 54 to 55. Extracted total RNA was treated by DNase I (Sigma-Aldrich, St. Louis, MO, USA) to eliminate genomic DNA, and first-strand complementary DNA (cDNA) was synthesized from 1 μg total RNA using a M-MLV cDNA Synthesis Kit (Enzynomics, Daejeon, Korea). PCR was conducted with the primers specific for six genes including ar, lh, cyp17a1, sox9, star, and β-actin under the following condition: initial denaturation step (94 °C for 5 min), cycling step (30 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 45 s), and the final extension step (72 °C for 10 min). The PCR products mixed with LoadingSTAR (DyneBio, Seongnam, Korea) were size fractionated by 1.2 % (w/v) agarose gel (Lonza, Rockland, ME, USA) electrophoresis and visualized with a UV transilluminator. The primer sequences are listed in Table 1.
Primer sequences for ar, cyp17a1, lh, sox9, and star genes were referred from Berbejillo et al. (2012)
ar androgen receptor, cyp17a1 cytochrome P450, family 17, subfamily a, polypeptide 1, lh luteinizing hormone, sox9 sry-box containing gene 9, star steroidogenic acute regulatory protein
To investigate cell viability after freezing and thawing, 2 × 105 cells from each cell line at passages 52 to 57 were suspended with freezing solution consisting of L15 containing 10 % (v/v) dimethyl sulfoxide (DMSO; Sigma-Aldrich) and 20 % (v/v) FBS, and the cell suspensions were transferred into 1.2-mL cryovials (Corning, Corning, NY, USA). Subsequently, the cryovials were frozen in pre-chilled freezing containers (Nalgene, Rochester, NY, USA) with the cooling rate of −1 °C/min. After 12 h in a deep freezer at −75 °C, the cryovials were stored in liquid nitrogen (−196 °C) for 24 to 27 days. The cells were thawed in a 37 °C water bath for 2 min, 30 s and harvested by centrifugation. The post-thaw cells were suspended with culture media, and 1 × 104 cells were seeded in 96-well microplates. Cell viability was measured using CCK-8. Non-frozen cells were used as a control, and cell viability was calculated as absorbancesample/absorbancecontrol × 100. This experiment was conducted in triplicate.
The Statistical Analysis System (SAS Institute, Cary, NC, USA) program was used to analyze the effect of each treatment. When analysis of variance (ANOVA) detected a significant main effect, treatments were analyzed subsequently by Duncan’s method. Significant differences among treatments were defined by a p value <0.05.
Results
Five gonad-dissociated cells derived from five individuals were subjected to in vitro culture. All cell populations were attached to substrata after 1 day of culture and reached 90 to 100 % confluency in 4 to 6 days through continuous proliferation. They were subcultured continuously, and 1 × 106 cells of each were frozen at least once before the 10th passage. Each cell line was named as ABG1, ABG3, ABG5, ABG6, and ABG7 (Table 2). Up to date, all the five cell lines have been cultured stably and constantly over at least the 73th passage and 402 culture days. For preservation and future use, all cell lines were cryopreserved in liquid nitrogen with a sufficient number of vials containing each cell lines at an interval of about 5 passages (Table 2).
aComposition of culture media was Leibovitz’s L-15 medium supplemented with 20 % (v/v) fetal bovine serum and 1 % (v/v) penicillin and streptomycin
bAll cell lines were frozen and stored in liquid nitrogen (−196 °C)
To decide optimal growth conditions for A. baerii gonad-derived cell lines, the effects of temperature and FBS concentration on cellular growth were evaluated. As shown in Fig. 1, similar effects of temperature on cellular growth were observed in the three cell lines tested. Significant high cell growth was detected at both 28 and 30 °C (p < 0.05), and it was decreased as temperature goes down. In 24 °C, the lowest cell growth was observed, but the cells kept attaching on the substrata, maintained normal morphology, and grew slowly. To test the effects of FBS concentration on cellular growth, culture temperature was fixed at 28 °C according to the result of temperature test. As expected, absence of FBS in the culture did not induce both cell growth and attachment in all the cell lines tested and growth rate was increased in a dose-dependent manner. The ABG3 and ABG5 lines grew better significantly in the media containing 15 and 20 % FBS relative to the other media while the ABG1 line showed maximum growth in the media containing 20 % FBS (p < 0.05).
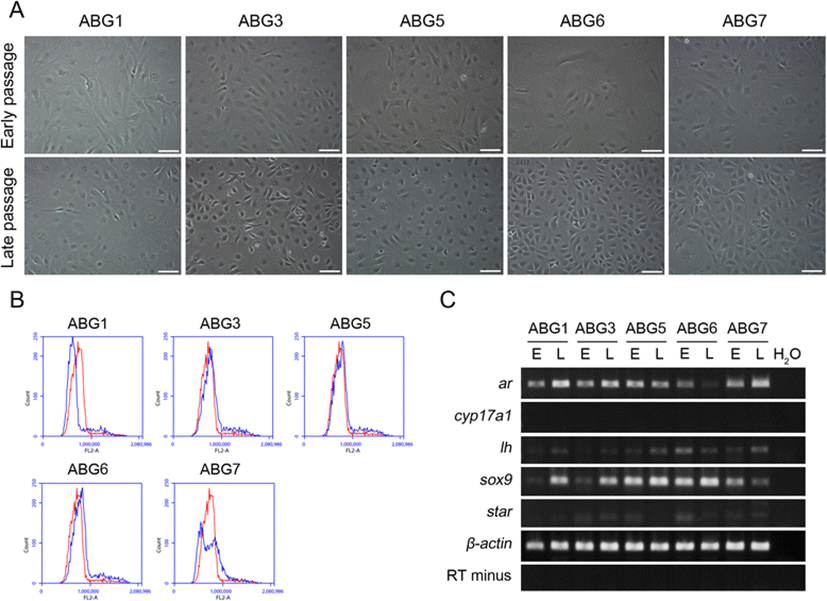
During the culture period, all the cell lines were composed of mainly two types of cells based on morphological criteria: wide-spread cells that occupy a wide surface area and small polygonal cells that grow densely. At an early passage (passage nos. 15 to 16), a ratio of wide-spread cells was relatively higher than that of small polygonal cells in all cell lines and a certain difference among cell lines was not observed visually. As the cell lines were subcultured consistently, a ratio of small polygonal cells was relatively increased and some differences among cell lines appeared at a late passage (passage nos. 54 to 55). The ABG1 and ABG5 lines have maintained a high ratio of wide-spread cells, whereas the ABG3 and ABG6 lines contained dominantly small polygonal cells. In the ABG7 line, two cell types were mixed at a similar ratio (Fig. 2a). To evaluate ploidy of the cell lines after long-term culture, DNA contents of each cell line at late passage were measured by flow cytometry. As shown in Fig. 2b, all the five cell lines showed diploid DNA contents similar to the control cell line despite long-term culture. Two peaks within diploid peak of the ABG7 line are seemed to be caused by the property of the ABG7 line in which two cell types existed at the same ratio. Next, RT-PCR analysis was conducted to identify the expression of a set of genes that are known to be expressed in A. baerii gonad tissue in the established cell lines at both early and late passages. As the results, the expression of ar, lh, and sox9 genes was maintained at late passage, more or less, in all the cell lines, but the cyp17a1 gene was not expressed at all in both early and late passages of all the cell lines. In case of the star gene, overall expression was weak with no expression in the ABG1 line and its expression in the ABG5 line was lost at late passage (Fig. 2c).
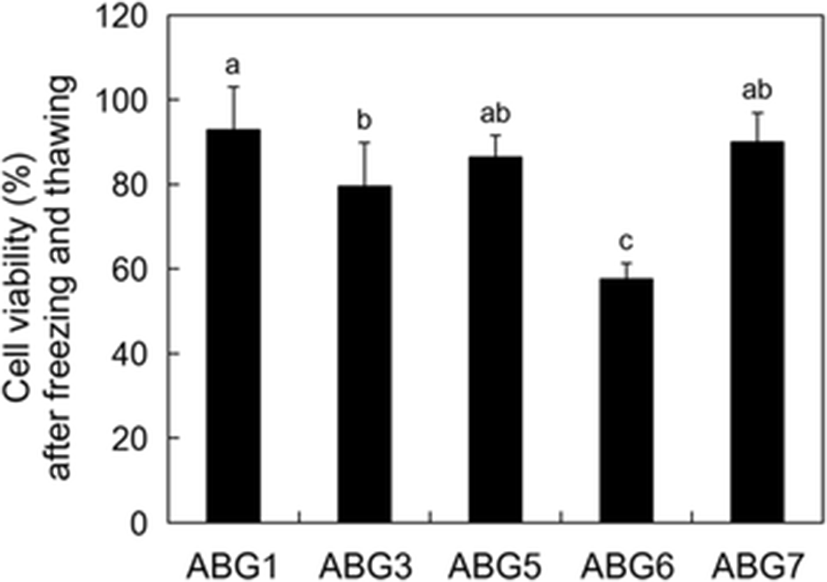
To evaluate the feasibility of cryopreservation of the established cell lines, the viabilities of post-thaw cells which were frozen and stored in liquid nitrogen (−196 °C) for 24 to 27 days were measured in each cell line. High post-thaw cell viabilities of more than 79.6 % were detected in four cell lines including ABG1, 3, 5, and 7 (92.9, 79.6, 86.4, and 90.0 %, respectively), but the ABG6 line showed significant low post-thaw cell viability of 57.6 % (Fig. 3; p < 0.05).
Discussion
In this study, we successfully established five A. baerii gonad-derived cell lines from five trials (success rate, 5/5 = 100 %) and all established cell lines could grow stably during a long period of more than 1 year without any significant growth retardation and marked deterioration in culture. Furthermore, these cell lines showed high post-thaw cell viabilities of more than 79.6 % except one line that showed 57.6 % post-thaw cell viability suggesting the feasibility of stable cryopreservation. These advantages of easiness in cell line derivation, long-term maintenance, and cryopreservation can maximize the availability of these cell lines as the feeder cells for culturing A. baerii germline stem cells. Culture of most stem cells requires continuous supply of feeder cells (Evans and Kaufman 1981; Matsui et al., 1992; Kanatsu-Shinohara et al., 2003; Takahashi and Yamanaka, 2006; Jing et al., 2010; Pacchiarotti et al., 2010). Thus, in case of the feeder cells that have a relatively short lifespan like mouse embryonic fibroblasts that are feeder cells for culturing human and mouse embryonic stem cells, sacrifice of animals and labor-intensive work for cell preparation should be conducted repeatedly (Amit et al., 2003). Unlike this, the cell lines that can grow long-term and be stored stably are free to such limitations. In addition, limitation of using A. baerii as an endangered species also can be overcome by the advantages. On the other side, these cell lines are able to play a role in universal biological studies such as toxicology and drug discovery, functional studies for genes and proteins, and study for cell-pathogen interaction (Lakra et al., 2011). Indeed, there is a report that conducted virus susceptibility test on Chinese sturgeon A. sinensis tail fin cell line to verify the feasibility of fish virus culture and isolation using a cultured cell line (Zhou et al., 2008).
For cell culture, we used 28 °C culture temperature and the media containing 20 % FBS based on our previous report that cultured A. baerii heart-derived cell lines (Kim et al., 2014) and the results from the experiment in this study. However, other conditions also can be attractive based on our results. We found that the established cell lines could be cultured in a wide range of culture temperatures from 24 to 30 °C even though significant high cell growth was observed in 28 and 30 °C conditions. Culture in various temperatures may have a potential merit as feeder cells because co-culture with germline stem cells may require a different optimal culture temperature as in the case of mammalian species (Lee et al., 2013). In addition, the results showed that 15 % FBS also induced significant high cell growth at a similar level with 20 % FBS in the ABG3 and ABG5 lines, suggesting that the application of a cell line-specific protocol can save overall cost by diminishing the use of high-cost FBS.
In general, tissue-derived primary cell populations in culture undergo a selection process by various factors such as cellular damage in isolation, cell attachment, limitations in nutrient or substrates, and growth competition between different cells. Thus, one or two cell types are eventually remained when a cell line forms in most case (Freshney, 2010). Likewise, we observed that two cell types were dominant in the culture of A. baerii gonad-dissociated cells in morphological criteria. At the initial stage of culture, it looked that wide-spread cells were more predominant than small polygonal cells but the proportion of one of the two cell types was increased as the culture was progressed in all the cell lines except the ABG7 line that showed a similar ratio of the two cell types. This indicates that both cell types can be easily adapted in the culture environment used in this study and they are the major targets for further study. In another aspect, two cell types may represent each cell population at different differentiation stages in a common cell lineage.
All cell lines showed diploid DNA contents at passages 54 to 55, indicating that all cell lines maintained an original normal state without cell transformation featured by aneuploidy or heteroploidy (Freshney, 2010). Moreover, the genes expressed at early passage were still expressed at late passage in all except the star gene of the ABG5 line. Although the data is very limited in terms of the number of genes tested, this result implies that the cell lines maintained their original characteristics in some degree at least up to passages 54 to 55. Taken together, these findings suggest that the A. baerii gonad-derived cell lines might be able to maintain their primary supportive role in germline stem cells in spite of long-term culture.
To establish gonad-derived cell lines, we used a 1-year-old A. baerii of which sex cannot be indentified externally (Keyvanshokooh and Gharaei, 2010). Unfortunately, we could not carry out histological inspection of gonad tissues from which the cell lines were originated due to a lack of available sample. Moreover, because there is no marker to discriminate sex at a cellular level in this species, sex of the cell lines established in this study is not clear. Therefore, it remains to be demonstrated if which germline stem cells, female or male, the established cell lines can support better. In previous reports, in vitro culture of germline stem cells requires several growth factors such as fibroblast growth factor (FGF), glial cell-derived neurotrophic factor (GDNF), leukemia inhibitory factor (LIF), or stem cell factor (SCF) (Matsui et al., 1992; Kubota et al., 2004; Zhang et al., 2011 in mammalian; Hong et al., 2004; Wong et al., 2013; Li et al., 2014 in fish). These growth factors can be supplied by feeder cells or exogenously. However, because commercially available growth factors are usually of human or mouse origin, supply of them by feeder cells seems to be more appropriate in fish germline stem cell culture (Wong et al., 2013). In this study, we did not identify the expression of the growth factors in the cell lines due to the absence of available information about them. Considering the importance of the growth factors in supporting germline stem cells in vitro, identification of their expression should be checked in future studies. Otherwise, the cell lines can be manipulated artificially so as to express stably such growth factors.
Conclusions
This study reports the establishment of five A. baerii gonad-derived cell lines that stable long-term culture and cryopreservation were possible. These cell lines can be utilized as a basic material to develop the culture system for germline stem cells in sturgeon species as well as a tool for general biological studies.