Background
Heavy metals are considered major anthropogenic contaminants in marine environments, as they pose a serious threat to marine organisms due to their toxicity, persistence, and bioaccumulation tendencies (DeForest et al. 2007). Chromium is an essential microelement in insulin-related functions, but it is also a toxic element. A typical feature of chromium in the environment is that it does not disappear but merely changes its form and valence. Demirak et al. (2006) reported that the gills are the major site for Cr accumulation due to their close relationship with the external environment. Hexavalent chromium (Cr6+) induces histological alterations in fish, including hyperplasia of the gill epithelium and fusion of the secondary gill lamella. However, the liver plays a major role in the detoxification of metals through the induction of metal-binding proteins such as metallothionein (MT) (Linde et al. 2005). MT, a metal-binding protein, is a low molecular weight cysteine-rich protein that not only plays an important role in the transport and storage of essential metals but also provides protection against the toxic effect of metals (Lange et al. 2002). In aquatic species, exposure to metal increases the MT level (Lange et al. 2002). The MT induction in fish is known to be high in tissues directly involved in metal uptake, storage, and excretion, such as the gill, liver, kidney, intestine, and muscle (Amiard et al. 2006). The toxic effects of Cr are widely believed to be associated with the stimulation of free radical processes as well as the formation of highly reactive intermediates of Cr6+ reduction (Lushchak et al. 2008). Reactive oxygen species (ROS) which are generated during Cr6+ degradation exert oxidative stress in cells (Wang et al. 2004). As a result, the biological system induces antioxidants such as superoxide dismutase (SOD), catalase, and glutathione (GSH)-related enzymes to combat the increased levels of ROS. Antioxidant enzymes serve as excellent biomarkers to study oxidative stress in aquatic organisms. Roberts and Oris (2004) investigated a series of biomarkers in rainbow trout, Oncorhynchus mykiss, in response to Cr toxicity. Arillo and Melodia (1998) also pointed out that Cr6+ induces alterations in the oxidative function of mitochondria in trout. Furthermore, the intracellular fate of both essential and non-essential metal ions strongly depends on thiol-containing molecules, particularly GSH and MT (Schlenk and Rice 1998). Therefore, the objectives of this study were (a) to investigate Cr6+ accumulation in tissues of the mullet Mugil cephalus, after waterborne exposure and (b) to investigate the effects of exposure to Cr6+ on MT and GSH levels including glutathione S-transferase (GST) and SOD activities in the liver and gills to elucidate the cause and effect relationship between metals and antioxidant responses.
Methods
Mullet M. cephalus (weight, 48.25 ± 8.36 g; length, 17.23 ± 0.85 cm) were collected from an aquafarm in Ha-Dong, South Korea, and were acclimatized to laboratory conditions for 4 weeks before experimentation. The water quality parameters measured for the bioassay were as follows: pH, 8.10 ± 0.2; salinity, 33.50 ± 0.6 ‰; DO, 7.14 ± 0.3 mg L−1; and chromium, ≤0.1 μg L−1. All experiments were conducted in a seawater temperature of 20 ± 0.5 °C under a 12-h light/12-h dark cycle. Experimental fish were exposed to waterborne treatments of 0, 25, 50, 100, 200, and 400 μg L−1 Cr6+ concentrations for 4 weeks. Potassium dichromate (Sigma-Aldrich, Inc., USA) was used as the test compound, and the culture water was renewed every 2 days. Fish were anesthetized using benzocaine, and the liver, gill, kidney, spleen, and muscle were sampled and kept at −80 °C until analysis after exposures of 2 and 4 weeks.
Each tissue was lyophilized to constant weight and then digested using the wet digestion method. Lyophilized organs were dissolved in HNO3 and re-dried by heating to 120 °C. Final samples were dissolved in 2 % HNO3 and used for analysis after filtration (Advantec MFS, Inc., 0.2-μm filter). The concentration of total chromium in tissues was measured using an ICP-MS (Elan, PerkinElmer, Inc., USA) equipped with an automatic sampler using argon gas. The accumulation factor (AF) is measured by the following formula: AF = [Me]fw,exp − [Me]fw,control/[Me]water, where [Me]fw,exp, [Me]fw,control, and [Me]water are the metal concentrations in the experimental group, control group, and water, respectively, measured in micrograms per gram (Holwerda 1991).
The liver and gill tissues used to determine enzyme activity were rinsed in 0.1 M KCl (pH 7.4) and homogenized (099CK4424, Glas-Col, Germany) in 0.1 M PBS (pH 7.4). The homogenate was centrifuged at 10,000×g for 60 min (+4 °C), and the supernatant was used for GST and SOD assays. To determine MT and GSH, tissues were homogenized in 20 mM Tris buffer (pH 7.8) containing 0.25 M sucrose. The homogenate was centrifuged at 10,000×g for 30 min (+4 °C), and the supernatant was used for assays. The supernatant was stored at −75 °C prior to analysis. Protein concentrations were determined using the method of Bradford (1976), with bovine serum albumin as a standard. MT was measured at 412 nm following the method of Viarengo and Nott (1993) using 0.25 M NaCl, 1 N HCl containing 4 mM EDTA, and 0.2 M sodium phosphate solution containing 0.43 mM 5,5-dithiobis-2-nitrobenzoic acid (DTNB). GSH was measured at 412 nm following the method of Srivastava and Beutler (1970) using a precipitation solution containing metaphosphoric acid, disodium ethylenediamine tetraacetic acid (Na2EDTA), 0.3 M Na2HPO4 solution, and 0.5 mM DTNB. The GSH level was evaluated and determined using the reduced glutathione standard curve. The SOD activity was measured at 450 nm using an SOD Assay Kit-WST (Dojindo Co., Japan) to determine the 50 % inhibitor rate of the reduction of 2-(4-lodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-1). The SOD activity at the 50 % inhibitor rate was expressed as units per milligram protein. GST activity was measured using 0.2 M potassium phosphate (pH 6.5), 10 mM GSH, and 20 μL of 10 mM CDNB. This enzyme assay was according to the methods of Habig et al. (1974) with minor modifications (Anosike et al. 1991). GST activity was determined by an absorbance increase at 340 nm after 5 min and was expressed as nanomoles per minute per milligram protein. For statistical analysis, a one-way analysis of variance (ANOVA) was used followed by Duncan’s multiple range tests using SPSS statistical software (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant when P < 0.05.
Results and discussion
Cr6+ accumulation in the organs of the mullet depending on the exposure time and exposure dose are shown in Fig. 1. As evidenced, the Cr6+ accumulation resulted in a net increase of the total Cr6+ content in all organs except the muscle with respect to the control. At 400 μg L−1, the liver, kidneys, intestine, and gill showed similar Cr6+ contents; however, these four organs statistically differed from the control. The accumulation patterns of Cr6+ after 4-week exposure occurred in the following order: kidney ≈ liver > intestine ≈ gill > spleen > muscle. Similar patterns of metal accumulation have also been demonstrated for other aquatic animals (Kim et al. 2004). Metal accumulation in the organs of fish is dependent upon the exposure time and exposure dose as well as other factors, such as temperature, age, interaction with other metals, water chemistry, and metabolic activity of the fish (Campana et al. 2003). Here, Cr6+ accumulation in the mullet kidney was higher than that in the liver except at 400 μg L−1, indicating that Cr6+ accumulation in the kidney was more effective than that in the liver of the mullet. Similar results have been reported in the freshwater fish Cirrhinus mrigala, exposed to chromium (Palaniappan and Karthikeyan 2009); in catfish Ictalurus punctatus, exposed to mercuric chloride (Kendall 1977); and in carp (Cinier et al. 1999) and eel (Yang and Chen 1999) exposed to cadmium. Kraal et al. (1995) observed that the accumulation of metal in the kidney was higher than that in the liver during chronic cadmium exposure. The main location of metal accumulation varies strongly across fish species. In addition, the maintenance of high accumulation in the intestine and kidney has often been observed, as these tissues comprise the principal route of excretion for most toxicants. In the present study, gill tissue contained a substantial amount of Cr6+ during the experimental period (Fig. 1). Demirak et al. (2006) and Malik et al. (2010) have indicated that the gills are highly Cr-accumulating organs in fish due to their close relationship with the external environment. On the other hand, the concentrations of Cr6+ were lower in the muscles compared to the other organs examined in this study. This result is particularly important because the muscles contribute the greatest mass of the flesh that is consumed as food. Table 1 presents the AFs for various organs after Cr6+ exposure in the mullet. The AFs increased with exposure period and were inversely related to the exposure concentration in the organs of the mullet. The AFs were calculated for two major purposes: first, to measure how much Cr6+ is accumulated with respect to aqueous exposure concentration, and second, to determine the finite limit in the ability of fish to accumulate metals (Sorensen 1991). Similar patterns have also been observed in carp (Cinier et al. 1999), eel (Yang and Chen 1999), and olive flounder after cadmium exposure (Kim et al. 2004).
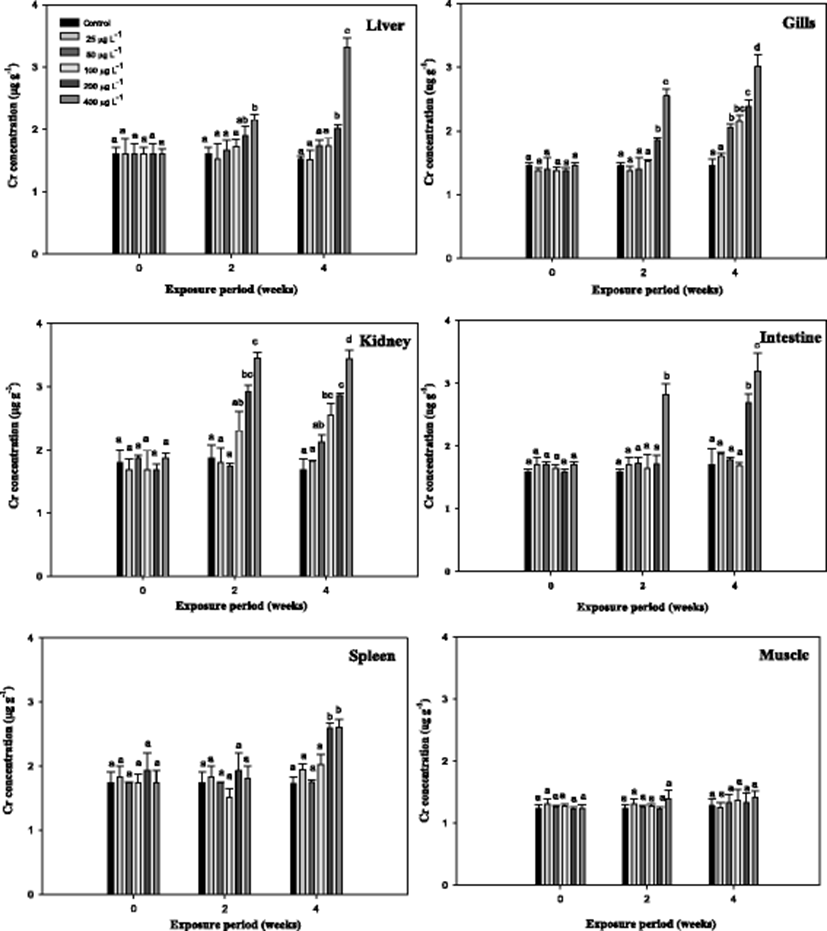
The results of metallothionein (MT) in the mullet are presented in Fig. 2. The liver MT levels were significantly increased compared with the control with exposure period. However, the gill MT level did not show the significance from the control (Fig. 2). We observed that Cr6+ concentrations were the highest in the liver of the mullet, which was consistent with the findings of Çogun et al. (2006), who demonstrated that the liver is the main target for heavy metals. The high value of bioaccumulation observed in the liver reflects the affinity of the metal to these tissues. Because the liver is a major producer of metal-binding proteins, MT can be closely related to heavy metal exposure. In accordance with metal residues, Fig. 2 indicates that Cr6+ induces hepatic MT in the mullet. Hepatic MT synthesis is induced by cytokines and stress hormones and in fish by bivalent metals, including cadmium, zinc, copper, lead, and mercury. In mullet, MT is predominantly a Cu-binding protein (Linde et al. 2005), which is similar to other conditions of hepatic Cu overload (Klein et al. 1997). In this study, the level of gill MT was not as high as that in the liver and did not significantly differ from the controls in the mullet (Fig. 2). In the rainbow trout O. mykiss, hepatic and gill MT mRNA levels significantly increased over 7 days of Cr6+ exposure (Roberts and Oris 2004). These authors reported that although gill MT expression was not as high in the liver, expression occurred much earlier in the experiment (Roberts and Oris 2004). The gill, which lies in direct contact with the water column, would have greater interaction with Cr ions; however, in this study, the hepatic tissues exhibited a much greater capacity for production of MT than the gill. Furthermore, Roberts and Oris (2004) suggested that a greater amount of Cr3+ likely reaches the liver than the gill, thus inducing the liver to produce greater amounts of MT. MT has also been recognized as being involved in cellular antioxidant functions (Sato and Bremner 1993).
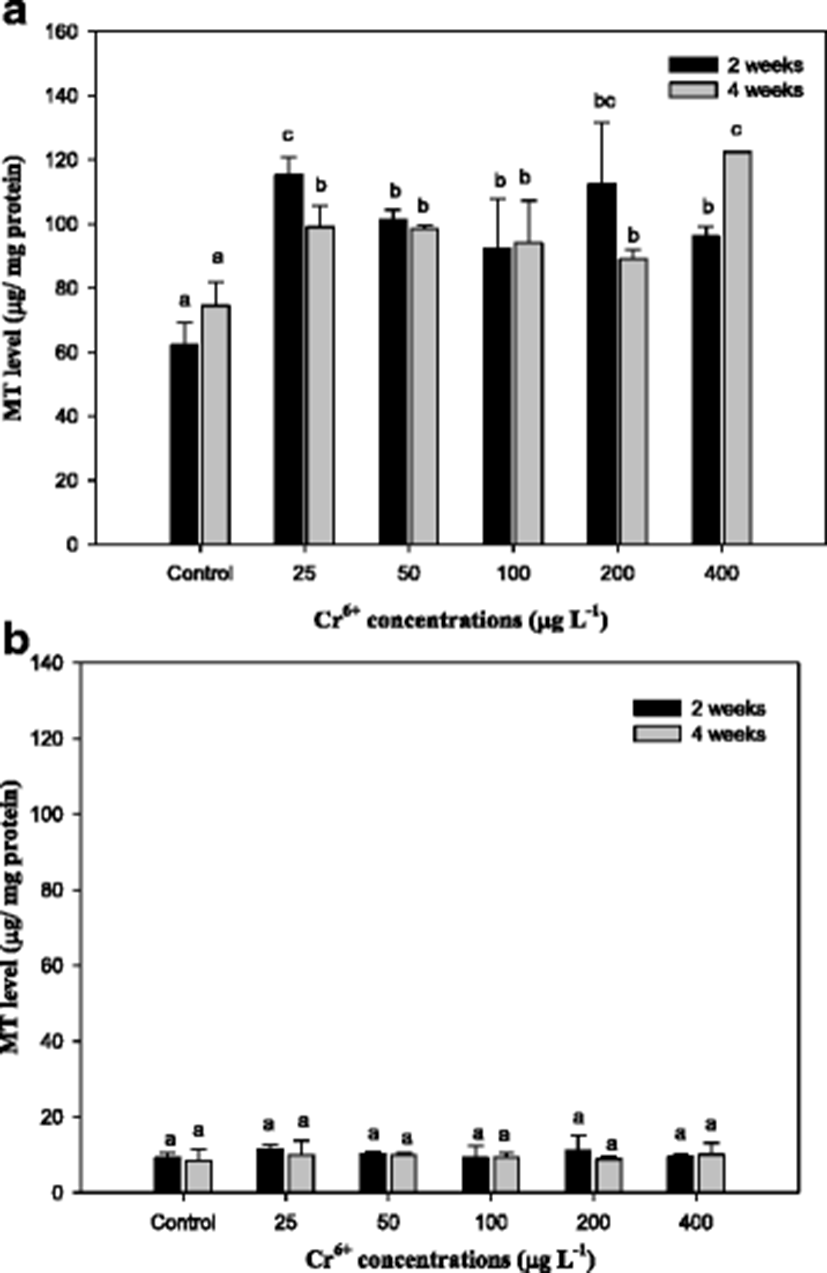
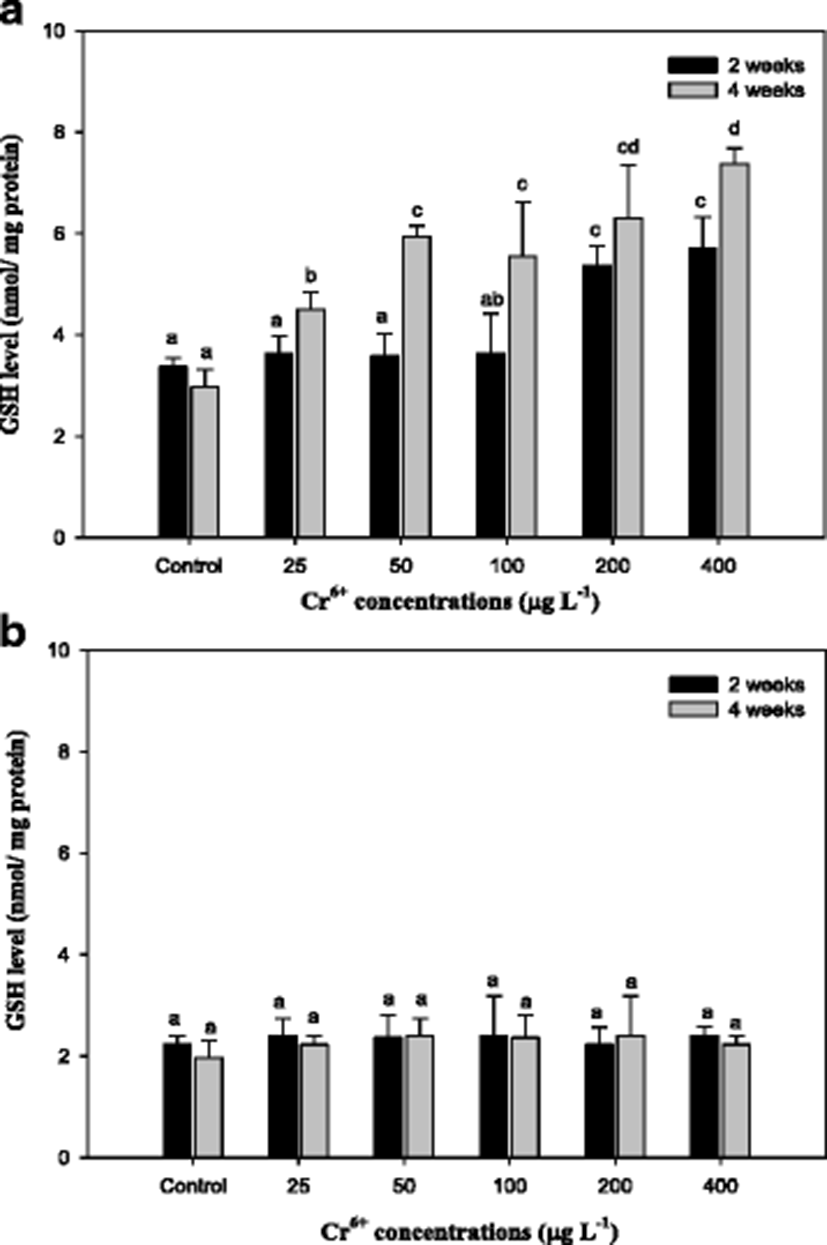
The capacity for Cr6+ conversion to Cr3+ in biological systems may be the mechanism of detoxification of Cr6+ (Lushchak et al. 2008). The results of reduced glutathione (GSH) in the mullet are reported in Fig. 3. As seen, the GSH level in the liver with Cr6+ exposure significantly increased. GSH is considered a first line of cellular defense against metals by chelating and detoxifying the metals, scavenging oxyradicals, and participating in detoxification reactions catalyzed by glutathione peroxidase (Thomas and Wofford 1984; Potter and Tran 1993; Sies 1999). Sengupta et al. (1990) showed that the acute oral administration of Cr to rats led to an increase of lipid peroxide and decreases in GSH, GST, and SOD of intestinal epithelial cells, whereas a chronic one led to increases in lipid peroxide, SOD, and GPx activity and to a decrease in GST activity. Exposure to heavy metals caused a time- and dose-dependent increase of GSH in various fish species, including mullet (Thomas and Wofford 1984; Lange et al. 2002; Zirong and Shijun 2007; Atli and Canli 2008). Atli and Canli (2008) concluded that the induction of GSH is probably due to the primary defense system for protecting the fish from oxidative stress. Similar results in this study indicated that exposure of the mullet to Cr6+ led to a significant increase in hepatic GSH levels, and these changes were associated with the exposure period and concentrations of Cr6+ (Fig. 3). However, we observed no changes in GSH in gill tissue. Kubrak et al. (2010) found that the gills of the goldfish Carassius auratus treated with Cr6+ did not result in any changes in GSH after 96 h and only the liver of goldfish C. auratus exhibited an increase in GSH. Consequently, we noted that GSH, including MT, in the mullet gill was not affected by Cr6+ treatment. It reflects the mullet liver has more responsibility for the detoxification of toxic effects of Cr6+ than the gills.
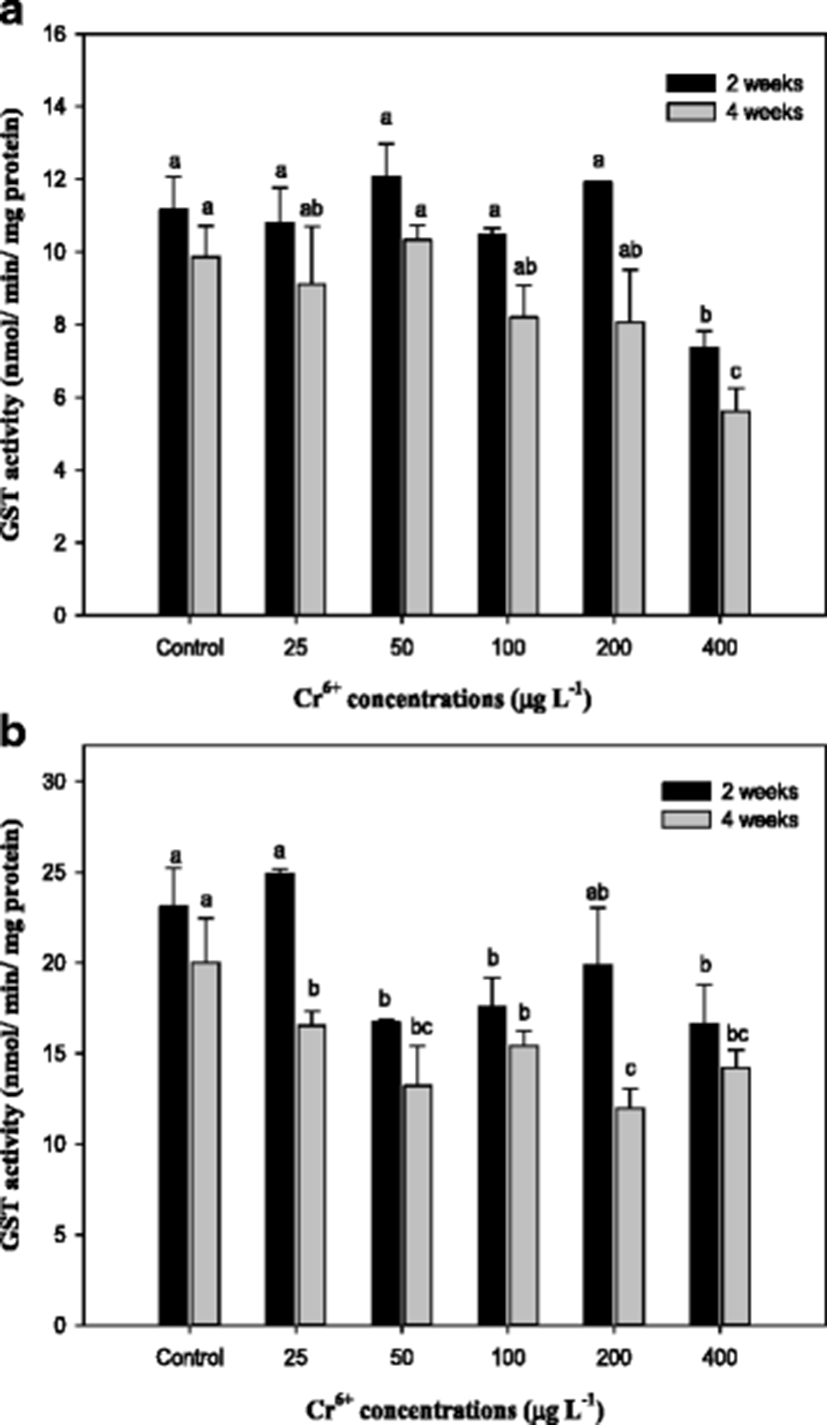
Glutathione S-transferase (GST) activities in the mullet liver and gills exposed to Cr6+ for 4 weeks were significantly reduced compared with those in the control as shown in Fig. 4. Similar results have been reported in previous studies addressing the effect of Cr on GST in goldfish C. auratus (Lushchak et al. 2009a, b; Kubrak et al. 2010). Elia et al. (2000) reported that high mercury concentrations induced a reduction of GST that was likely responsible for the increased hepatic GSH levels in catfish Ictalurus melas. GST is a well-known phase-II enzyme of the metabolism of detoxification, and it conjugates GSH to certain xenobiotic compounds or to their metabolites (Kim and Kang 2015). GST and GSH are important in protecting organisms from oxidative stress, and the fluctuation of GSH in organisms exposed to metals appears to be generally accompanied by variation in GST activity (Paris-Palacios et al. 2000).
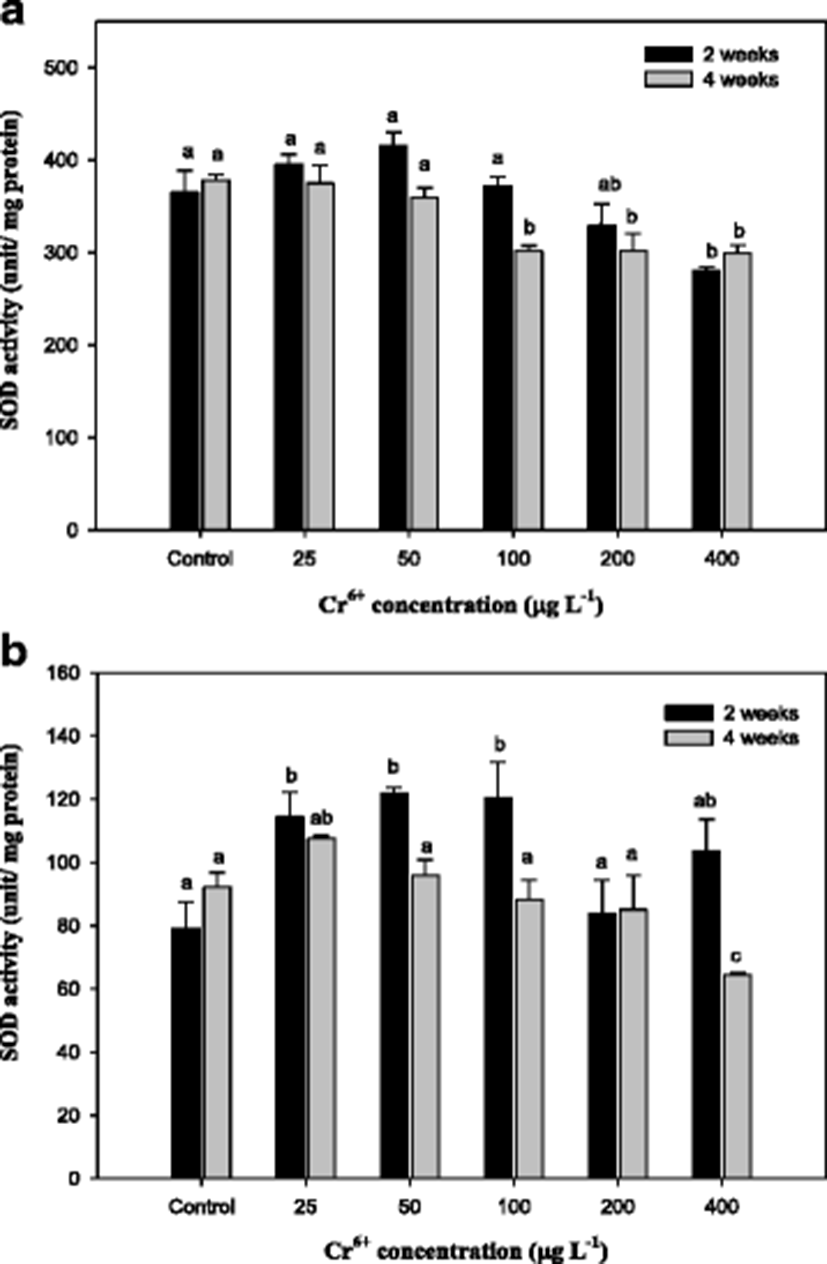
Superoxide dismutase (SOD) activity in the mullet exposed to Cr6+ for 4 weeks is presented in Fig. 5. Significant changes were observed in the liver in only 400 μg L−1 at 2 weeks and 100–400 μg L−1 at 4 weeks compared with the control. In the gills, SOD activity was significantly decreased in 400 μg L−1 at 4 weeks. Wang et al. (2004) demonstrated that SOD completely abolished ROS generation induced by Cr6+. Roberts and Oris (2004) also suggested that the generation of ROS by the reduction of Cr6+ to Cr3+ occurred to a greater extent in the gill than the liver and that the gill was more sensitive than the liver to Cr toxicity. In this study, gill SOD activity in the mullet significantly increased at concentrations of 25–100 μg L−1; however, liver SOD activity was significantly reduced at 100–400 μg L−1 (Fig. 5). Kubrak et al. (2010) reported that a 48-h exposure to Cr6+ reduced SOD activity in the brain of goldfish C. auratus and had no effect on liver SOD. Roberts and Oris (2004) demonstrated that SOD activity in rainbow trout, O. mykiss, significantly increased in the liver but did not change in the gill after Cr6+ exposure.
Conclusions
In conclusion, results from these studies indicate that a high concentration of Cr-mediated oxidative stress could inactivate SOD, although Cr induces a tissue-specific antioxidant response. These results indicated that significant modulation of the activities of these biomarkers occurred in response to Cr6+ toxicity. This study highlighted that Cr6+ treatment at a level ≥200 μg L−1 may affect bioaccumulation and that the AF increased with exposure time in the mullet. The responses of bioaccumulation, MT induction, and GSH to Cr6+ exposure were all very likely to be interconnected. Furthermore, this study demonstrated that Cr6+ exposure decreased the markers of oxidative stress, SOD and GST, in the mullet liver and gill.