Background
Marine algae, which are abundant in the coast areas over the world and very popular as food in Korea and Japan, can be a useful source of therapeutic compounds. Ishige okamurae, a brown alga, has been found throughout the temperate coastal zone of the Jeju Island, South Korea (Ahn et al. 2011). Yoon et al. (2009) has reported antioxidant secondary metabolites, such as phloroglucinol, 6,6′-bieckol, and diphlorethohydroxycarmalol (DPHC) in I. okamurae. In particular, DPHC, one of the main compounds in I. okamurae, has been reported to exhibit diverse biological effects, such as antioxidant (Heo et al. 2008; Zou et al. 2008), anti-inflammatory (Kim et al. 2009), antihypoglycemic (Heo et al. 2009), and antiviral effects (Ahn et al. 2006). However, thus far, except DPHC, other main antioxidant compounds from I. okamurae have not been sufficiently investigated. Hence, 2,2′-azino-bis(3-ethylbenzo thiazoline-6-sulphonic acid) (ABTS+) online HPLC was employed for the detection of the various main antioxidant compounds, such as DPHC, from I. okamurae.
Typically, ABTS+ has been employed for measuring the antioxidant activity of compounds from several natural products (Koleva et al. 2001; He et al. 2010). The determination of antioxidant activity was based on the decrease in absorbance at 680–730 nm after the reaction of HPLC-separated antioxidants with ABTS+, which forms a deep green color by reaction with potassium persulfate and loses its color by reaction with an antioxidant compound (Re et al. 1999; Amanda et al. 2005). This reagent applied to ABTS+ online HPLC systems can detect unknown compounds which exhibit antioxidant activities from the crude extracts of algae or plants (Koleva et al. 2000; Koleva et al. 2001; Lee et al. 2013a). ABTS+ online HPLC is simple and effective for detecting the main antioxidant compounds from various compounds and exhibits the following advantages: use of HPLC with cost-effective reagents, time savings, and a non-laborious experimental method (Koleva et al. 2001; Lee et al. 2013a). ABTS+ online HPLC has also been previously employed for the detection of phenolic compounds (e.g., gallic acid, 3-caffeoylquinic acid, and epigallocatechin gallate) from green tea (Amanda et al. 2005).
In general, the isolation and purification of DPHC from I. okamurae require various complex processes, such as the use of silica gel, Sephadex-LH 20 column chromatography, and preparative HPLC (Heo et al. 2009). However, these conventional methods exhibit several drawbacks, in that they are time-consuming, they require limited amounts of compounds, as well as target compounds are irreversibly adsorbed on the stationary phase during separation (Lee et al. 2014a). Because of these drawbacks, a preparative centrifugal partition chromatography (CPC) system might be a useful technology. Preparative CPC is liquid–liquid separation without the use of a support, based on the dispersion of each component in two non-mixed liquid phases (Lee et al. 2013a; Jeon et al. 2016). This principle makes it possible to isolate large amount of compounds with purities of greater than 90 % in a single step (Berthod et al. 1988; Delannay et al. 2006; Lee et al. 2013a, Bourdat-Deschamps et al. 2004). In addition, the CPC system provides various technological merits, such as a short operation time, inexpensive product, higher yields and throughput, and reduced operating costs (Berthod and Armstrong 1988; Lee et al. 2013a). Hence, in this study, we searched and largely isolated the antioxidant compounds from the ethyl acetate fraction of I. okamurae (IOEA) by ABTS+ online HPLC and single-step of preparative CPC.
Methods
The brown alga, I. okamurae, was collected from Seongsan located in the eastern part of Jeju Island, South Korea. The sample was rinsed more than three times with tap water to remove the surface matters such as epiphytes, salt, and sand and then stored in a refrigerator at −20 °C after cautiously washing with tap water. Thereafter, the frozen sample was freeze-dried before extraction.
All solvents used for the preparation of the crude sample for CPC separation were of analytical grade (Daejung Chemicals & Metals Co., Seoul, South Korea), and HPLC grade solvents were purchased from Burdick & Jackson (MI, USA).
The powdered I. okamurae (500 g) was extracted three times with 70 % ethanol (EtOH) under stirring for 24 h at room temperature, then it was filtered. The filtrated extract was concentrated under decompression and freeze-dried to powder. The powdered extract (68 g) was then mixed in water (1.0 L) and in a row separated with n-hexane (n-Hex), chloroform (CHCl3), and ethyl acetate (EtOAc).
HPLC coadunate with 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay was progressed using the method of Lee et al. (2013a, 2013c) with some modifications. For the HPLC, an Atlantis T3 column (3 μL, 3.0 × 150 mm column) (Waters, USA) was used, and the mobile phase was acetonitrile-water in gradient mode as follows: (0 → 40 min, 5:95 → 50:50 v/v; ~50 min, ~100:0 v/v; ~70 min, ~100:0 v/v). The UV absorbance was detected at 230 nm, and the flow rate was 0.2 mL/min.
HPLC–DAD–ESI/MS analyses were carried out using a Hewlett–Packard 1100 series HPLC system equipped with a binary pump, a degasser, an autosampler, a DAD detector, and a column oven (Hewlett–Packard, Waldbronn, Germany) coadunate to a Finnigan MAT LCQ ion-trap mass spectrometer (Finnigan MAT, San Jose, CA, USA). The MS was fitted with a Finnigan electrospray source and can analyze ions up to m/z 2000. Xcalibur software (Finnigan MAT) was used for MS operation. The chromatographic conditions are equal to those described in the “ABTS+ online HPLC assay” section, and the flow cell outlet was combined to a splitting valve, from which a flow of 0.2 mL/min was diverted to the electrospray ion source via short fused silica tubing. Negative ion mass spectra of the column emission were recorded in the range m/z 100~2000. The source voltage was set to 4.5 kV and the capillary temperature to 250 °C. The other conditions were as follows: capillary voltage, 36.5 V; inter-octapole lens voltage, 10 V; sheath gas, 80 psi (551.6 kPa); and auxiliary gas, 20 psi (137.9 kPa).
The CPC operations were progressed using the method specified by Lee et al. (2013a, 2014a). The CPC operations were progressed using a two-phase solvent system which was composed of n-hexane:EtOAc:MeOH:water (0.5:10:4:6, v/v). The bottom aqueous phase was used as the stationary phase, whereas the top organic phase was employed as the mobile phase. When the mobile phase appears from the column, it reaches a hydrostatic equilibrium (back pressure, 3.1 MPa), and then the emission from the CPC process is monitored in UV at 254 nm. And fractions of 6 mL were collected in test tubes by a fraction collector (FC 203B, Gilson, South Korea).
Results and discussion
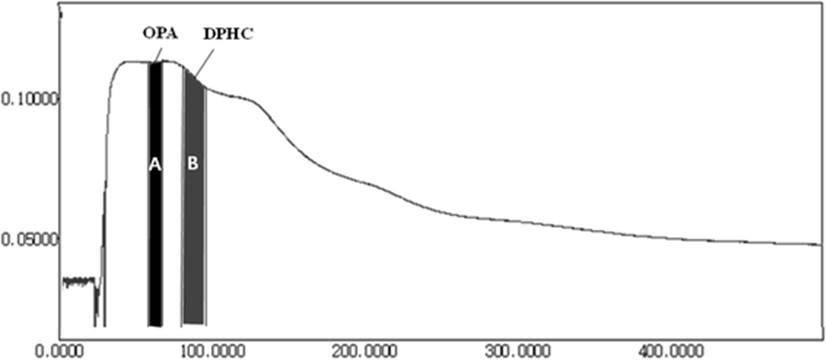
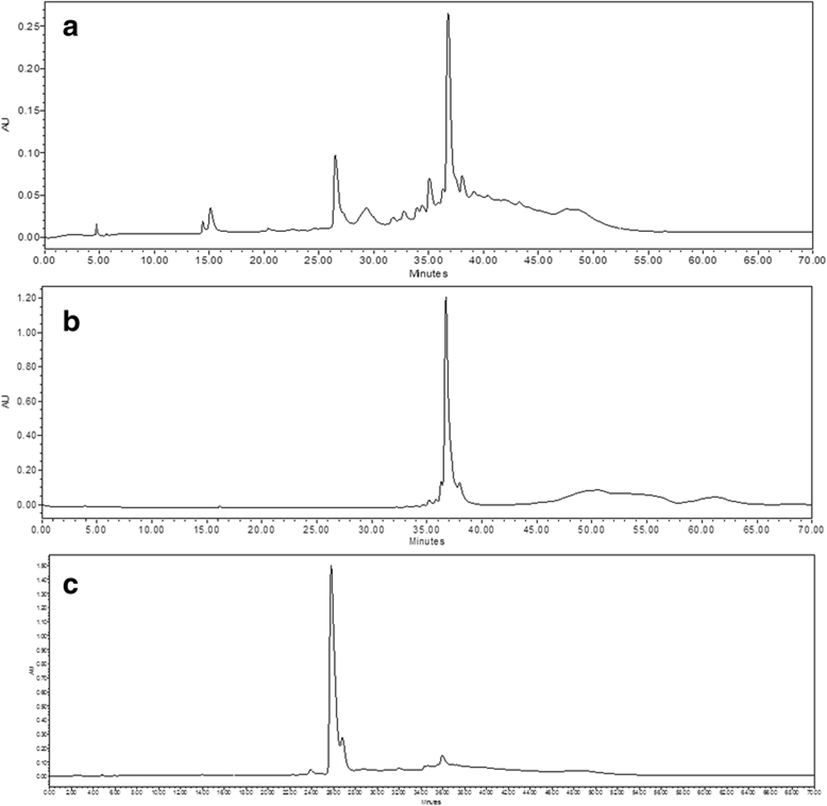
Because the EtOAc fraction of I. okamurae has been reported to be rich in antioxidant compounds, such as phloroglucinol, 6,6′-bieckol, and DPHC (Yoon et al. 2009), it was selected herein. From the results obtained, the two main antioxidant compounds from IOEA were rapidly and easily identified by ABTS+ online HPLC as a measure of the decrease of absorbance of ABTS+ at 680 nm (Fig. 1). Antioxidant activity was determined by online HPLC based on the decrease of absorbance at 680–730 nm after the antioxidants were separated by the post-column activation of HPLC with ABTS+ (Lee et al. 2016). The two main antioxidant compounds were confirmed to be DPHC (molecular weight (MW) 512, C24H16O13) and octaphlorethol A (OPA) (MW 994, C48H34O24) by LC-DAD-ESI/MS (Piao et al. 2013; Lee et al. 2014b) (Fig. 2).
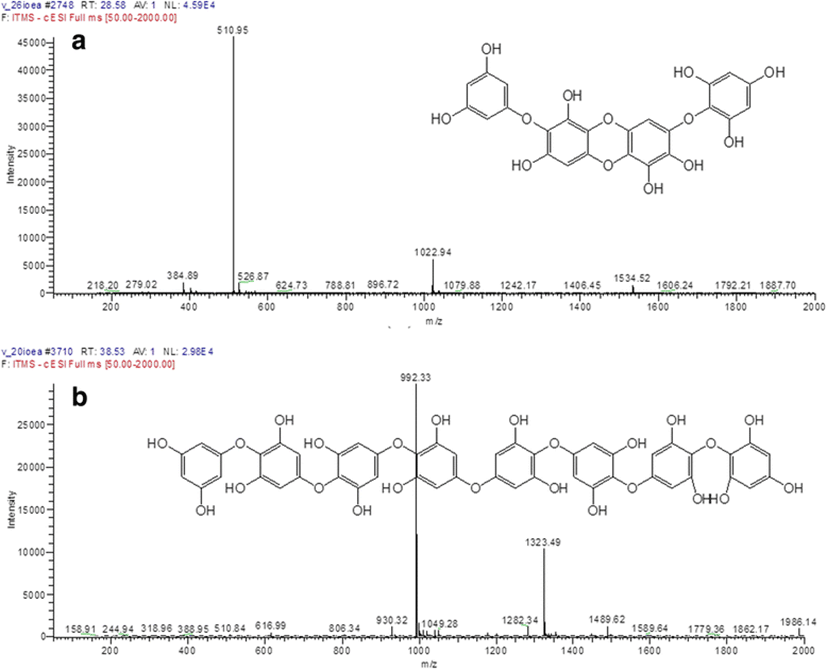
DPHC, one of the most important biological compounds from I. okamurae, has been reported to demonstrate protective effects against radiation-induced cell damage in mice (Ahn et al. 2011), inhibitory activity of α-glucosidase and α-amylase in diabetic mice (Heo et al. 2009), and potential preventive effect of Alzheimer’s disease by the inhibition of acetyl- and butyrylcholinesterase (Yoon et al. 2009). OPA has only been isolated from Ishige foliacea; however, in our study, OPA was first confirmed from I. okamurae (Kang et al. 2014; Lee et al. 2012; Lee et al. 2013b). Generally, algae of the same genus and/or algae with similar phenotype have been reported to produce similar secondary metabolites (Hillis 1962; Ha et al. 2015). In particular, phlorotannins such as eckol, dieckol, and 6,6′-bieckol present in Ecklonia cava are also produced by Eisenia bicyclis, which has a phenotype similar to that of E. cava (Kwon et al. 2013). Recently, OPA has been reported to exhibit protection against high-glucose-induced oxidative damage in vitro and in vivo (Kang et al. 2014), inhibit α-MSH-stimulated induced melanogenesis via the extracellular-signal-regulated kinase (ERK) pathway in B16F10 melanoma cells (Kim et al. 2013), increase glucose transporter 4-mediated glucose uptake in skeletal muscle cells (Lee et al. 2012), as well as demonstrate antihyperglycemic effects in streptozotocin-induced diabetic mice (Lee et al. 2014b). Hence, DPHC and OPA are very useful materials in the nutraceutical industry. However, for their industry, it is difficult to largely isolate and purify DPHC and OPA from I. okamurae and I. foliacea, attributed to the complex processes. Accordingly, we investigate the optimum protocol for their efficient isolation and purification by preparative CPC.
In CPC, it is imperative to choose a suitable two-phase solvent system for separation (Lee et al. 2016). The partition coefficient (K) is calculated by the solubility of target compounds in a two-phase non-mixed solvent (Tayar et al. 1991; Jeon et al. 2014). The K values for an optimum separation condition are in the following range: 0.2 ≤ K ≤ 5.0 (Lee et al. 2014). Especially, the difference between the K values of the peaks of a target compound should be greater than 1.5–2 times of the K value. For selection of optimal solvent condition for isolation of DPHC and OPA in IOEA, their K values were calculated by utilizing various volume rates of two non-mixed solvents such as n-Hex:EtOAc:MeOH:water (v/v) (Table 1). The K values for 0.5:10:5:5 n-Hex:EtOAc:MeOH:water (v/v) were within the reported optimal range of the K value (0.2 ≤ K ≤ 5.0); however, a marginal difference was observed between the K values of DPHC (0.97) and OPA (0.99). In addition, the K values (DPHC, 10.42; OPA, 6.46) obtained with 1:9:1:9 n-Hex:EtOAc:MeOH:water (v/v) were not in the optimal range. Hence, 0.5:10:4:6 n-Hex:EtOAc:MeOH:water (v/v), with K values of 1.62 and 2.71 for DPHC and OPA, respectively, was utilized as the best condition to isolate the two compounds, because of a difference of 1.67 times between the K values of both the compounds. Thus, the preparative CPC system consisting of two descending and ascending modes was employed; when K value is more than 1, it is selected as the ascending mode (Lee et al. 2016). Finally, we operated the CPC system under both the best solvent condition and ascending mode (mobile and stationary phases represent the top and bottom phases, respectively).
|
Solvent condition |
DPHC |
OPA |
|
|---|---|---|---|
|
n-Hex:EtOAc:MeOH:water |
1:9:1:9 |
10.42 |
6.46 |
|
n-Hex:EtOAc:MeOH:water |
0.5:10:4:6 |
1.62 |
2.71 |
|
n-Hex:EtOAc:MeOH:water |
0.5:10:5:5 |
0.97 |
0.99 |
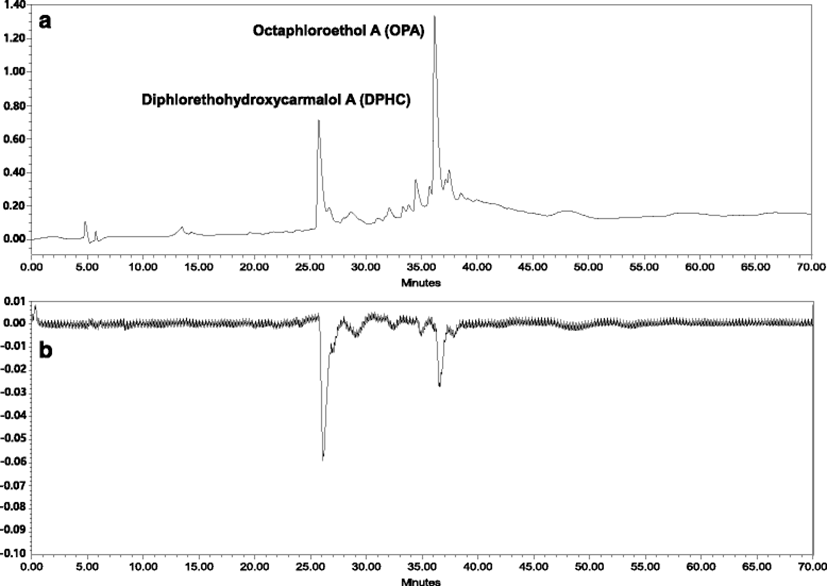
DPHC and OPA were efficiently and simply separated from IOEA according to the optimum K value applied; CPC chromatogram is shown in Fig. 3. DPHC and OPA were confirmed to be present in fractions A and B, respectively, by HPLC LC-DAD-ESI/MS analysis (Fig. 4). The amounts of DPHC and OPA purified from IOEA (500 mg) using a single-step CPC system were 39 mg (7.8 % yield) and 23 mg (4.6 % yield), respectively. However, the previous reported yield of DPHC from the ethyl acetate fraction of I. okamurae (15.3 g) was relatively low (275.8 mg; approximately 2 %) (Ahn et al. 2011) as compared with the result obtained by this study. Lee et al. (2014a) have isolated 31.1 mg of phlorofucofuroeckol-A (6 % yield) and 40.2 mg of dieckol (8 % yield) from 500 mg of the EtOAc fraction of E. cava by CPC. On the other hand, Ahn et al. (2007) have only obtained a product yield of less than 1 % by conventional isolation methods.