Background
Protein is the basic component of all animal tissues, and it constitutes about 65–75 % in fish tissues on a dry matter basis (Wilson 2002). Dietary protein has great impact on the growth and body composition of fish (Lovell 1989) because it provides the essential amino acids for body protein synthesis as well as energy for growth and maintenance. However, protein is one of the most expensive major nutrients in fish feeds, and its inclusion in fish diets has significant effects on operational costs in aquaculture (NRC 1993). It is well documented that increase of dietary protein can lead to improved fish production especially in the case of carnivorous fish species. However, the excess level of dietary protein will be used for energy and will lead to an increase in ammonia excretion which ultimately deteriorates the fish culture water that may be harmful for fish growth (Catacutan and Coloso 1995; Tibbetts et al. 2000). Furthermore, an inadequate level of dietary protein in aqua feeds can result in the stunted growth of fish. But the objective of proper feed formulation is to produce cost-effective feeds with maximum fish production at a minimum protein level (Halver and Hardy 2002). Therefore, it is imperative to determine the optimum dietary protein level in aquaculture diets for achieving cost-effective maximum growth of fish together with improved culture environment.
Barred knifejaw, Oplegnathus fasciatus, belonging to the family Oplegnathidae, is a popular food fish and an economically important cage aquaculture species in Korea as well as in East Asia (Meng et al. 1995). In 2014, its aquaculture production in Korea was approximately 884 metric tons (National Statistical Office 2014) which mostly came from cage aquaculture. It has high market value and consumer demand. The dietary protein requirements of several important aquaculture fish species have been determined including channel catfish, Ictalurus punctatus (Garling and Wilson 1976); Asian sea bass, Lates calcarifer (Catacutan and Coloso 1995); Indian major carp, Labeo rohita (Das et al. 1991); Nile tilapia, Oreochromis niloticus (El-Sayed and Teshima 1992); olive flounder, Paralichthys olivaceus (Kim et al. 2005); Korean rockfish, Sebastes schlegelii (Kim et al. 2004), and Japanese eel, Anguilla japonica (Okorie et al. 2007); black sea bass, Centropristis striata (Alam et al. 2008); black sea bream, Sparus macrocephalus (Zhang et al. 2010); and silver pomfret, Pampus argenteus (Hossain et al. 2010). For most of the cultured species, dietary protein requirement has been found to be between 30 and 55 % of the diet depending on the species, fish size, dietary protein sources, and environmental condition (Hepher 1988; NRC 1993). Therefore, it is important to estimate dietary protein requirements in fish under different conditions (Luo et al. 2004). In a previous study, Kang et al. (1998) reported that the optimum dietary protein and lipid levels for parrot fish or barred knifejaw are 46 and 16 %, respectively, in a protein and lipid ratio experiment in tanks. However, for the first time, in this study, we aimed to re-evaluate the optimum dietary protein level in diets for the juvenile barred knifejaw with a fixed dietary energy level in cage culture condition, which is widely being practiced in Korea.
Methods
Composition of the experimental diets is shown in Table 1. Five experimental diets containing white fish meal and casein as the main protein sources were formulated with protein levels of 35, 40, 45, 50, or 60 % at the expense of α-potato starch and squid liver oil. The diets were formulated to be isocaloric, containing 18.8 kJ/g energy based on calculation (Garling and Wilson 1976; NRC 1993). In the diets, wheat flour and α-potato starch were used as carbohydrate sources to adjust the energy content of the experimental diets. Carboxymethylcellulose (CMC) of high viscosity was used as binder. The experimental diets were also fortified with vitamin and mineral premixes (Table 1). The actual nutrients and amino acid contents in experimental diets are shown in Table 2. All ingredients were mixed and pelleted by a pelleting machine without heating using a 2-mm-diameter module (Baokyong Commercial Co., Busan, Korea). After air drying for 48 h, all the pellets were broken up, sieved into the proper pellet size, sealed, and kept at −20 °C until used.
aSuhyup feed Co. Ltd., Uiryeong, Korea
bUnited States Biochemical, Cleveland, OH, USA
cYoung Nam Flour Mills Co., Busan, Korea
dEwha Oil Co., Ltd., Busan, Korea
eContains (as mg/kg in diets) ascorbic acid, 300; dl-calcium pantothenate, 150; choline bitartrate, 3000; inositol, 150; menadione, 6; niacin, 150; pyridoxine.HCl, 15; riboflavin, 30; thiamine mononitrate, 15; dl-α-tocopherol acetate, 201; retinyl acetate, 6; biotin, 1.5; folic acid, 5.4; B12, 0.06
fContains (as mg/kg in diet) Al, 1.2; Ca, 5000; Cl, 100; Cu, 5.1; Co, 9.9; Na, 1280; Mg, 520; P, 5000; K, 4300; Zn, 27; Fe, 40.2; I, 4.6; Se, 0.2; Mn, 9.1
gVitamin C: l-ascorbyl-2-monophosphate, 35 % ascorbic acid activity (Hoffmann La Roche, Switzerland)
Values are means of duplicate samples of each diet
Juvenile barred knifejaw, O. fasciatus, were transported from Geoje Marine Hatchery (Geoje, Korea) of the National Fisheries Research and Development Institute, Republic of Korea, to Youngchang Fisheries Farm (Tongyeong, Republic of Korea). Before commencement of the experiment, barred knifejaw were acclimated in a circular concrete tank containing 5000 L water where they were fed commercial diet for 2 weeks at the fish farm. A feeding trial was conducted in a rectangular concrete tank (5 m × 5 m × 3 m, W × L × H) having a flow-through system, containing 15 floating net cages (each size: 60 cm × 40 cm × 90 cm, W × L × H) in triplicates of each experimental diet. Flow rate was adjusted to ensure adequate circulation of seawater. Supplemental aeration was provided to maintain dissolved oxygen levels near saturation. Water temperature was maintained 19 °C at the beginning of the feeding trial and was 22 °C at the end of the feeding trial according to the normal changes of natural water temperature. Twenty fish weighing 7.1 ± 0.06 g (mean ± SD) were randomly distributed to each net cage. Fish were fed one of the experimental diets twice (0900 and 1800 h) a day to apparent satiation at the rate of 4 % of wet body weight in the first 4 weeks and 3 % in the second 4 weeks. Total body weight of the fish in each cage was determined every 2 weeks, and the feed amounts were adjusted accordingly. The experiment was conducted under the guidelines of the Animal Ethics Committee Regulations, No. 554, issued by Pukyong National University, Busan, Republic of Korea.
At the end of the feeding trial, weight gain (WG), feed efficiency (FE), specific growth rate (SGR), protein efficiency ratio (PER), hepatosomatic index (HSI), condition factor (CF), protein retention efficiency (PRE), energy retention efficiency (ERE), hematocrit (percentage of packed cell volume—PCV%), hemoglobin (Hb), and survival rate of juvenile barred knifejaw were determined (Table 3). After the final weighing, five fish were randomly collected from each aquarium and blood samples were obtained using heparinized syringes from the caudal vein and pooled for blood hemoglobin and hematocrit determination (Brown 1980). For the determination of HSI, liver weight was taken by dissecting the fish. Crude protein, moisture, and ash of whole-body samples were analyzed by AOAC methods (1995). In brief, samples of diets and fish were dried to a constant weight at 135 °C for 2 h to determine moisture content. Ash was determined by incineration using a muffle furnace at 550 °C for 3 h. Crude lipid was determined by Soxhlet extraction unit using the Soxtec System 1046 (Foss, Hoganas, Sweden), and crude protein content was analyzed by Kjeldahl method (N × 6.25) after acid digestion. Amino acids were measured with an automatic amino acid analyzer (S433; Sykam, Gilching, Germany).
Values are means from triplicate groups of fish where the means in each row with a different superscript are significantly different (P < 0.05)
1Percent weight gain: (final wt. − initial wt.) × 100/initial wt
2Feed efficiency: (wet weight gain/dry feed intake) × 100
3Specific growth rate: 100 × (ln final wt. − ln initial wt.)/days
4Protein efficiency ratio: (wet weight gain/protein intake) × 100
5Hepatosomatic index: (liver weight/body weight) × 100
6Condition factor: [fish wt. (g)/fish length (cm)3] × 100
7Protein retention efficiency: [(final total body protein − initial total body protein)/total dietary protein fed] × 100
8Energy retention efficiency: [(final total body energy − initial total body energy)/total dietary energy fed] × 100
All the data were subjected to one-way ANOVA using SAS version 9.1 software (SAS Institute, Cary, NC, USA) to test the effects of dietary protein levels (Zar 1984). When a significant treatment effect was observed, a least significant difference (LSD) test was used to compare the differences between treatment means (P < 0.05). Broken-line analysis (Robbins et al. 1979) was used to estimate the optimum level of protein requirement for juvenile barred knifejaw.
Results
The growth performance of barred knifejaw fed experimental diets at different protein levels is shown in Table 3. After 8 weeks of feeding trial, WG of the fish fed the 45, 50, and 60 % crude protein (CP) were significantly higher than those of the fish fed the 35 and 40 % CP diets (P < 0.05). However, there were no significant differences in WG among the fish fed the 45, 50, or 60 % CP diets. FE was significantly lower in the fish fed the 35 % CP than in the fish fed the 45, 50, and 60 % CP diets (P < 0.05). In contrast to WG and FE, PER and PRE decreased with increasing dietary protein levels. The highest and the lowest ERE values were observed at the 60 and 35 % CP levels, respectively. HSI was highest at the 35 % CP level, whereas the lowest HSI was observed at the 60 % CP level. CF followed the same trend like FE of fish at different protein levels. A significantly higher mortality was observed in the fish fed the 60 % CP diet compared to the fish fed the 35, 40, 45, and 50 % CP diets. In considering hematological characteristics of fish, the juvenile barred knifejaw fed the 35 % CP diet showed a significantly lower hemoglobin level than the fish fed the 40, 45, 50, and 60 % CP diets (P < 0.05). There were no significant differences in hematocrit levels among the fish fed the experimental diets (P > 0.05).
The whole-body proximate composition of juvenile barred knifejaw is shown in Table 4. The table shows that CP and crude lipid (CL) content in the whole body increased with the increase in dietary protein levels. Significantly higher whole-body CP content was found in the fish fed the 45, 50, and 60 % CP diets than in the fish fed the 35 and 40 % CP diets. Whole-body CL content was found highest in the fish fed the 50 % CP diet and lowest in the fish fed the 40 % CP diet. No significant differences were found in the fish fed the experimental diets in terms of whole-body ash and moisture contents.
Values are means from triplicate groups of fish where the means in each column with a different superscript are significantly different (P < 0.05)
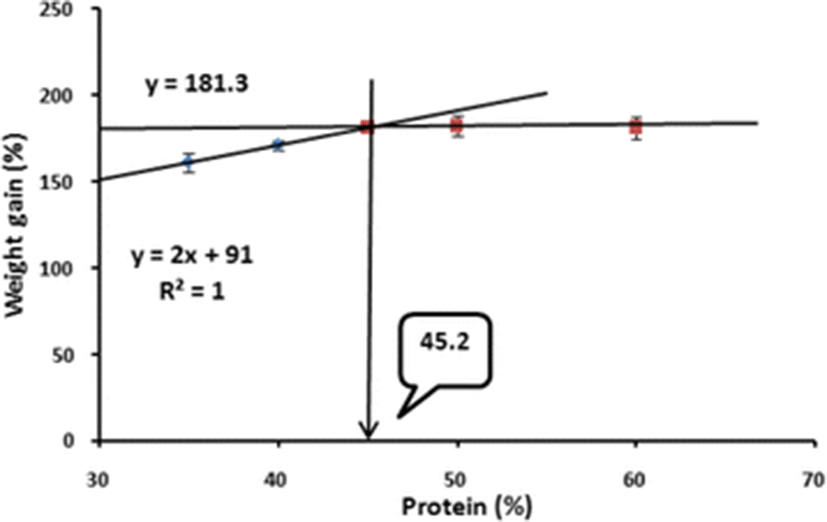
Broken-line analysis of WG of juvenile barred knifejaw indicated that the optimum dietary protein level was 45.2 % (Fig. 1).
Discussion
After 8 weeks of the feeding trial, ANOVA showed that WG of the fish fed the 45 % CP diet was significantly higher than those of the fish fed the 35 and 40 % CP diets but not significantly different from those of the fish fed the 50 and 60 % CP diets (Table 3). However, based on broken-line analysis of WG of barred knifejaw, the optimum dietary protein was 45.2 %. Similarly, Kim et al. (2004) reported that the optimum dietary protein level for Korean rockfish was 45.1 % CP based on broken-line analysis. In contrast to the present study, Kang et al. (1998) postulated that the optimum dietary protein level should be 46 % for the same species when reared in tank condition. Lovell (1972) reported that the protein requirement of fish varies on the culture environment. Hastings and Dupree (1969) found that channel catfish fed a practical-type diet containing 40 % protein showed linear growth in terms of WG in an aquarium system, whereas the same species of fish fed the same diet with 38 % protein showed linear WG in a pond. Moreover, Lovell (1972) proposed that in the cage culture system the required protein level could be 40 % for the same species of fish. Luo et al. (2004) reported that protein requirements for grouper fish species vary over a wide range between 40 and 60 % depending on the species and culture condition.
In line with our result, the protein requirement of black sea bass, C. striata, was found to be 45.3 % (Alam et al. 2008); 45 % for European sea bass, Dicentrarchus labrax (Peres and Oliva-Teles 1999); and 45 % for Florida pompano, Trachinotus carolinus (Lazo et al. 1998). However, in relation to the present study, the protein requirement levels were higher in olive flounder, P. olivaceus (46.4 %; Kim et al. 2002); grouper, Epinephelus malabaricus (47.8 %; Chen and Tsai 1994); and silver pomfret, P. argenteus (49 %; Hossain et al. 2010), and lower in hybrid striped bass, Morone chrysops × Morone saxatilis (40 %; Gatlin et al. 1994) and white bass, M. chrysops (41 %; Rudacille and Kohler 1998). Generally, when dietary protein levels increase, the growth of fish also increases (NRC 1993). In this experiment, WG, FE, SGR, and CF of fish improved with increasing dietary protein levels up to 45 % CP, and then, no further improvements were observed in these parameters at higher protein levels (Table 2). The trends were in agreement with Kim et al. (2004).
In the present study, there were clear reducing trends of PER and PRE with an increasing protein level in the treatment groups (Table 3). The result shows that possibly the dietary protein was efficiently utilized by fish for protein synthesis which is in agreement with Berger and Halver (1987). Similar results have been reported in other fish species (Bai et al. 1999; Kim et al. 2004; Kim et al. 2005; Hossain et al. 2010; Zhang et al. 2010). However, Kikuchi et al. (1992) and Lee et al. (2000) reported that PER values of olive flounder increased with increasing dietary protein levels. In another study, Dabrowski (1979) reported that the relationship between dietary protein and PER differs from species to species. In the present study, ERE increased with the increase of dietary protein levels which means dietary protein could be spared by non-protein energy sources. Dietary protein sparing helps to reduce feed cost and nitrogen waste outputs (Wang et al. 2006). Ng et al. (2008) reported that lipid plays an important role for protein sparing when the dietary protein level is low in relation to the requirement.
Hematological parameters like hemoglobin (Hb) and hematocrit (PCV) concentration levels were more or less similar in all the treatment groups (Table 3). A significantly lower amount of Hb was found in the blood of the fish fed the 35 % CP diet compared with the other experimental fish fed the higher levels of CP-contained diets. However, PCV concentration level was more or less similar in all the dietary treatment groups which may have resulted in no abnormalities in health status of the experimental fish. Kim et al. (2004) also found dietary protein levels have no significant effect on the hematological and serological characteristics of juvenile Korean rockfish. Hepatosomatic index (HSI) indicates the body condition of fish. In this study, HSI of barred knifejaw decreases with the protein level increases in all the dietary treatments which may indicate higher utilization of protein levels from the diets which is in agreement with Kim and Lall (2001). Survival rates in the fish fed the 60 % CP diet showed significantly higher mortality than those of the fish fed the 35, 40, 45, and 50 % CP diets possibly due to the production of a high level of nitrogenous wastes by the fish through higher fecal output which pollutes the culture environment (Catacutan and Coloso 1995; Tibbetts et al. 2000; Alam et al. 2008).
Proximate compositions in terms of moisture and ash contents of the fish fed the experimental diets were not significantly affected by dietary protein levels (Table 4) which are in accordance with Okorie et al. (2007) for juvenile Japanese eel and Kim et al. (2004) for Korean rockfish. In this experiment, the whole-body CP content increased with the increasing dietary protein levels which agree with the result found by Kim et al. (2002). Similarly, the body lipid content generally increased as the dietary protein level increased which is in agreement with Shiau and Lan (1996) for grouper and Bai et al. (1999) for yellow puffer. On the contrary, Kim et al. (2002) reported that as the CP content of the whole body increases, the whole-body CL content decreases.
Conclusions
In conclusion, based on broken-line analysis of WG, it can be corroborated that the optimum dietary protein level could be 45.2 % in juvenile barred knifejaw to achieve maximum growth when dietary energy content was fixed at 18.8 kJ/g diet. From this present finding, we assume that a cost-effective practical feed could be developed for the sustainable production of barred knifejaw in floating net cages.