Background
Acquired immunodeficiency syndrome (AIDS) was first reported in the USA in the year 1981. Since then, the number of AIDS patients and HIV-infected persons are persistently increasing worldwide. In 2015, the World Health Organization (WHO) reported that there are over 36.7 million AIDS patients worldwide (http://www.who.int/hiv/en/). To solve the problems resulting from AIDS, many investigations were conducted in many countries. Some well-known AIDS therapeutic agents such as 3′-azido-2′,3′-dideoxythymidine (AZT) and 2′,3′-dideoxyinosine (ddI) were approved by the Food and Drug Administration (FDA) and are now being administered to AIDS patients (Broder, 2010). However, these therapeutic agents are associated with many side effects such as anemia, neutropenia, and thrombocytopenia (Ay et al., 2013). Therefore, many research groups are being required to search for novel anti-HIV agents compatible to those of developed countries. Marine-based natural products that have chemically diverse and unique structures have received attention as sources for the development of new anti-HIV agents (Vo and Kim, 2010). Marine materials such as phlorotannins, sulfated chitoolifosaccharides, sulfated polysaccharides, and lectin have been reported to have anti-HIV activities. Additionally, several recent studies have reported that peptides from marine organisms can act as anti-HIV agents because of their therapeutic potential in the treatment of infectious diseases (Plaza et al., 2007 and 2009). In this regard, we examined anti-HIV activities of our previously isolated peptides from several marine organisms and found that a peptide from Spirulina maxima (SM-peptide) inhibits HIV-1 infection in human T cell line MT4. SM-peptide inhibited HIV-1IIIB-induced cell lysis, p24 antigen production, and HIV-1 reverse transcriptase.
Methods
We purified the S. maxima peptide as reported by Vo et al. (2014). The purity of the peptide was >98 % according to RP-HPLC assessment and N-terminal sequence analysis. The amino acid sequence of the final purified peptide was determined to be LDAVNR by electrospray ionization mass spectrometry (ESI/MS) (Vo et al., 2014).
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from Sigma–Aldrich (St. Louis, USA). DMSO was obtained from Amresco (Solon, USA). Specific antibodies for western blot were purchased from R&D systems (NE Minneapolis, USA) and Santa Cruz Biotechnology (Dallas, USA).
MT4 and H9/HIV-1IIIB cell lines were obtained from the NIH AIDS Reagent Program (Germantown, USA). All cell lines were cultured in RPMI-1640 (Thermo Scientific, USA) supplemented with 10% heat-inactivated FBS (Thermo Scientific, USA), 50 μg of streptomycin, and 50 U of penicillin per milliliter (PAA, USA) in 5 % CO2 containing air at 37 °C. Cells were passaged every 2–4 days and maintained at a cell density of 5 × 105–1 × 106 cells/ml. HIV-1IIIB viral particles were obtained from the supernatants of H9/HIV-1IIIB cell line. The viruses were stored at −80 °C until use. Viral titer was determined by p24 assay performed using MT4 cells. Viral titer was expressed as TCID50.
MT4 cells were seeded in a 96-well plate at 2 × 104 cells/well with RPMI-1640 medium containing 10 % FBS. After 24 h, the cells were treated with SM-peptide and incubated further for 24 h at 37 °C. Twenty-four hours later, fresh RPMI-1640 medium with 10 % FBS was added to each well. After 84 h, 20 μl of MTT solution (final concentration of 0.5 mg/ml) was added to each well and the plate was incubated for 4 h at 37 °C. Finally, 200 μl of DMSO was added to dissolve the purple formazan. The amount of formazan was determined by measuring absorbance at 595 nm using a microplate reader (Filter Max 5, Molecular Devices).
To determine the anti-HIV activity of SM-peptide on HIV-infected MT4 cells, MTT assay was performed. MT4 cells were seeded in duplicate in a 96-well plate at a density of 2 × 104 cells/well. After 24 h, stock virus of HIV-1IIIB was added to each well at 50 TCID50 along with the different concentrations of SM-peptide. The plate was incubated for 72 h at 37 °C with 5 % CO2. Cell viability was determined by MTT assay.
MT4 cells (2 × 104 cells/well) were seeded in a plate. After 1 day, the MT4 cells were treated with SM-peptide and infected with 50 TCID50 of HIV-1IIIB. The plate was incubated for 72 h. The supernatant was harvested by centrifugation. In order to determine the amount of HIV, Lenti-X p24 rapid titer kit was used according to the manufacturer’s protocol (Clontech, USA).
The activity of HIV-1 reverse transcriptase in the virus supernatant was determined by using a reverse transcriptase assay kit (Roche, Germany) according to the manufacturer’s protocol. Briefly, a reaction mixture containing poly(A) × oligo(dT)15 was added to the virus supernatant and incubated for 4 h at 37 °C. Two hundred microliters of anti-DIG-POD and ABTS were then added stepwise. The virus supernatant was incubated at room temperature until the color development was sufficient for detection. The absorbance of the virus supernatant was measured using a microplate reader at 405 nm.
Results
To identify natural peptides with anti-HIV-1 activity, we screened peptides from several marine organisms for their anti-HIV-1 activities. In this study, we showed that a peptide isolated from S. maxima, SM-peptide, has an anti-HIV-1 activity. The peptide was previously purified from enzymatic hydrolysates of S. maxima, and its sequence is LDAVNR with molecular mass of 686.37 Da (Vo et al., 2014).
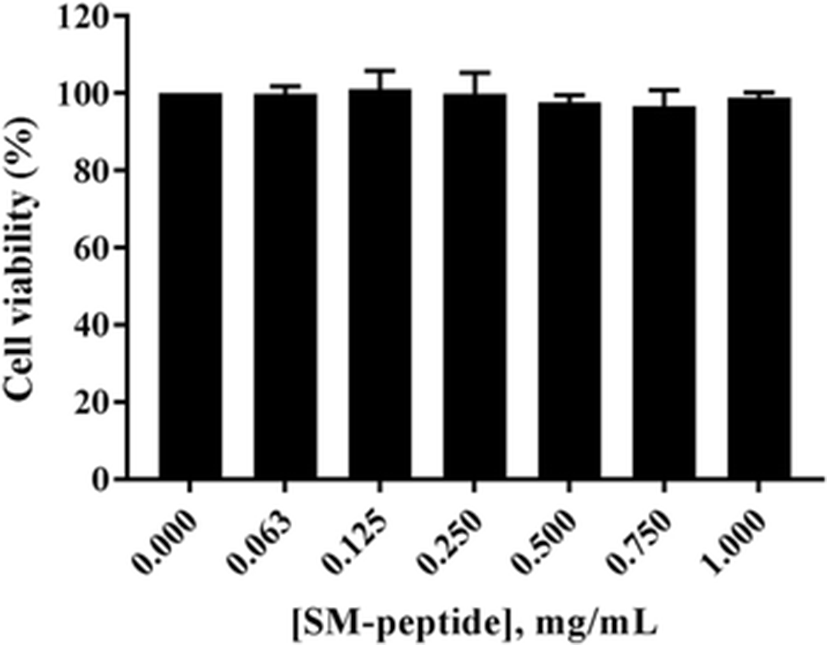
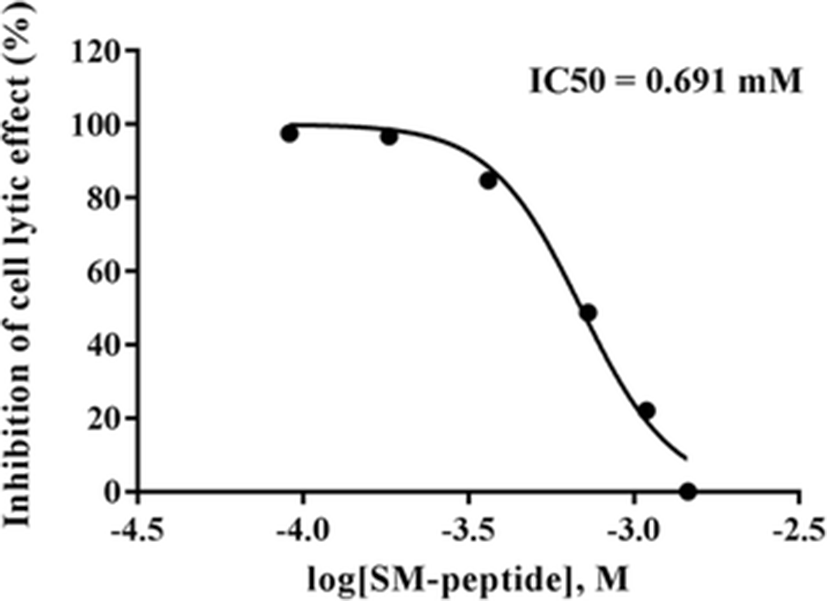
We first examined the cytotoxicity of SM-peptide in human MT4 T cells. The MT4 cells were treated with 0–1 mg/ml SM-peptide for 72 h, and the viability of MT4 cells was measured by a formazan-based MTT assay. Figure 1 shows that the SM-peptide did not affect the viability of MT4 cells at concentrations below 1 mg/ml (1.457 mM). Next, the protective activity of the SM-peptide on HIV-1IIIB-induced cell lysis was also investigated by MTT assay (Fig. 2). HIV-1IIIB-induced lysis of MT4 cells was decreased by SM-peptide. IC50 of SM-peptide against anti-HIV-1IIIB infection was assessed to be 0.475 mg/ml (0.691 mM).
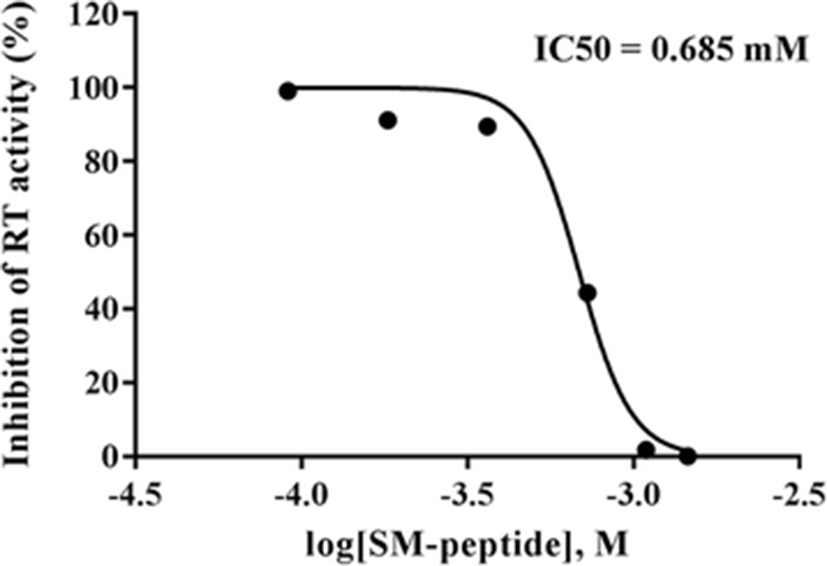
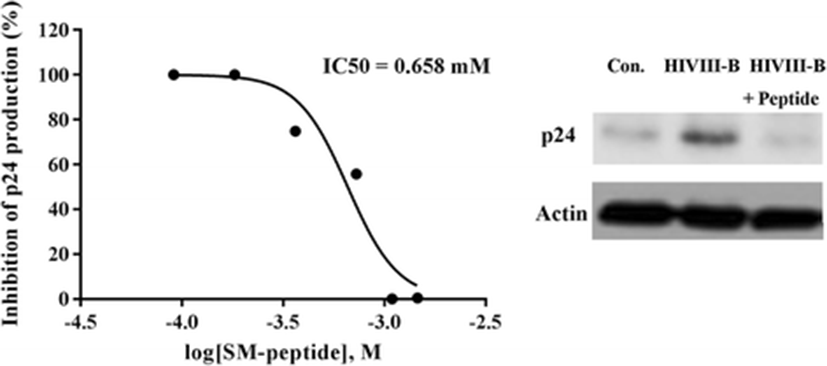
The anti-HIV-1 activity of SM-peptide was further examined by determining its effect on HIV-1 reverse transcriptase (RT) activity. RT activity is used by the HIV-1 retrovirus to convert single-strand genomic RNA into double-strand cDNA which can integrate into the host genome, potentially generating a long-term infection. SM-peptide was found to inhibit HIV-1IIIB-induced RT activation in MT4 cells (Fig. 3). SM-peptide (0.75 mg/ml, 1.093 mM) inhibited the RT activity in HIV-1IIIB-infected cells by approximately 90 % as compared to control (no peptide). In addition, we examined the effect of SM-peptide on HIV-1 p24 antigen production. p24 antigen is a structural protein that makes up most of the HIV viral core. High levels of p24 are present in the blood serum of newly infected individuals during the short period between infection and seroconversion, making p24 antigen assays useful in diagnosing primary HIV infection. As shown in Fig. 4, SM-peptide (0.75 mg/ml, 1.093 mM) inhibited the HIV-1IIIB p24 antigen production by more than 95 %. The inhibitory effect of SM-peptide on p24 protein production was also confirmed by western blot analysis of cell lysates with anti-p24 antibody. SM-peptide treatment decreased the HIV-1 p24 production significantly in cell lysates.
Taken together, these results suggest that marine natural SM-peptide inhibits HIV-1 infection by suppressing HIV-1IIIB-induced cell lysis, HIV-1 reverse transcriptase activity, and p24 antigen production.
Discussion
HIV, the causative agent of AIDS, is one of the hottest areas of medical research today. Because of the high prevalence and mortality associated with HIV infections, many researchers have focused on finding ways to fight the infection and improve the life span of HIV-infected individuals. So far, there are five major classes of anti-HIV-1 drugs that targeted distinct steps in the HIV life cycle: reverse transcriptase inhibitors, protease inhibitors, fusion inhibitors, integrase inhibitors, and multidrug combinations (Lifson et al., 2016). These drugs, though effective, do not cure HIV/AIDS. People with HIV infection still have the virus in their bodies and can spread the virus to others. Therefore, to improve the therapeutic potential of these medicines, identification of additional and suitably modified novel drug candidates is necessary (Migueles and Connors, 2015).
Numerous recent studies have reported that marine peptides from marine sponges may be used as anti-HIV agents (Vo and Kim, 2010; Ngo et al., 2012). The marine peptides have been found to mainly inhibit viral entry through membrane fusion (Plaza et al., 2007 and 2009; Zampella et al., 2008; Oku et al., 2004). The present study showed that a peptide isolated from S. maxima (SM-peptide) is noncytotoxic and inhibits HIV-1IIIB-induced cell lysis, reverse transcriptase activity, and viral p24 antigen production. SM-peptide was isolated and reported to downregulate the IgE receptor-mediated mast cell activation by Vo et al. (2014). HIV-induced immune dysfunction has been known to increase the likelihood of developing allergic and other immune-mediated diseases in patients. Antiretroviral therapy is associated with reconstitution of the immune system function. Therefore, SM-peptide may contribute to restore the body’s defense mechanism against infectious immune system disorder.
We tried to generate several derivatives of synthetic peptide to identify the specific amino acid residues responsible for the anti-HIV-1 activity of SM-peptide; however, we were unable to perform experiments in cells because of the insolubility of the synthetic peptides. Thus, the simulation methods such as the molecular docking approach may be useful to determine the mechanism of SM-peptide action on HIV-1 infection. In conclusion, SM-peptide is a novel and safe peptide with HIV-1 inhibitory activity and may be a promising candidate for the design of novel HIV/AIDS drugs.
Conclusions
We demonstrated that a peptide from S. maxima inhibits HIV-1 infection in a human T cell line MT4. SM-peptide exhibits inhibitory activity on HIV-1IIIB-induced lysis, p24 antigen production, and HIV-1 reverse transcriptase at noncytotoxic concentrations. This finding suggests that the marine peptide SM-peptide is a novel candidate peptide, which may be developed as a therapeutic agent for AIDS patients.