Background
Ammonia is one of the nitrogenous wastes especially in water. It is generated from the catabolism of amino acids, purines, and pyrimidines (Ruyet et al. 1995). In an aquatic environment, ammonia exists as two main forms such as unionized ammonia (NH3) and ionized ammonium (NH4+) (Randall and Tsui 2002). The toxicity of ammonia is significantly affected by the levels of pH; the increase in pH induces the concentration of NH3 increase (Richardson 1997). The toxic effects of ammonia exposure to aquatic animals strongly occur by the high concentration of unionized ammonium (NH3) because it can readily diffuse through the gill membranes (Sinha et al. 2012). Excessive ammonia can cause the growth performance decrease, tissue erosion and degeneration, immune suppression, and high mortality in aquatic animals, which acts as toxicity by increasing ammonia levels in blood and tissues (Lemarie et al. 2004; Li et al. 2014). In addition, the ammonia exposure also induces the neurotoxicity, oxidative stress, and oxygen delivery impairment as well as hyperactivity, convulsions, and coma (Wilkie 1997).
Ammonia toxicity can be affected by various environmental parameters like temperature, pH, salinity, and oxygen (Lemarie et al. 2004). Among the environmental indicators, temperature is one of the most major parameters to influence ammonia toxicity, and Richardson (1997) reported that the temperature increase caused a significant elevation in ammonia toxicity. Generally, the increase in temperature in aquatic animals induces the higher toxic effects under toxicity exposure (Patra et al. 2015), because the high temperature elevates the diffusion rate, bioavailability, and chemical reactions of the aquatic animals (Delos and Erickson 1999). Barbieri and Bondioli (2015) also reported the lower LC50 for the ammonia exposure of Pacu fish, Piaractus mesopotamicus by increasing water temperature, which means that the higher temperature causes the higher ammonia toxicity.
The exposure to toxic substances in aquatic environment can induce the negative effects on reproduction and growth performance in fish (Kim and Kang 2015). Among various toxicants, ammonia is one of the most toxic substances to cause the growth inhibition in fish farming, and the ammonia toxicity can be a main reason in fish mortality (El-Shafai et al. 2004). Given that the toxicants generally inhibit the growth performance in aquatic animals, the growth performance can be a good indicator to assess the toxicity in the animals.
Considering the exposure to toxicants induces the alterations of fish blood indicators, the hematological parameters can be a sensitive and reliable indicator to assess the toxicity on the exposed animals (Kim and Kang 2014). The ammonia exposure negatively causes the alterations in blood chemistry in aquatic animals as well as the decrease in reproductive capacity and growth rate (Vosyliene and Kazlauskiene 2004). Ajani (2008) reported a significant decrease in blood parameters such as red blood cell (RBC) counts, hemoglobin, and hematocrit of African catfish, Clarias gariepinus, exposed to ammonia, which may be due to anemia and haemodilution of haemolysis for RBC.
Rockfish, Sebastes schlegelii, is a commonly cultured fish in the marine net cages of South Korea because of its rapid growth performance and high demand, which is one of the three largest cultured fish in South Korea. But, the study about the ammonia exposure depending on water temperature has insufficiently been conducted, although the ammonia is ubiquitous in the marine net cages and highly toxic to cultured animals. Therefore, the purpose of this study was to evaluate the toxic effects for the ammonia exposure depending on water temperature to the S. schlegelii on hematological parameters and plasma components.
Methods
Rockfish, S. schlegelii (mean length 14.53 ± 1.14 cm and mean weight 38.36 ± 3.45 g), were obtained from a commercial farm (Tongyeong, Korea). Fish were held for 3 weeks in seawater at 19 °C to ensure that all individuals were healthy and feeding, and also to reset the thermal history (19 and 24 °C) of the animals prior to initiating temperature acclimations (temperature; 19.0 ± 0.6 and 23 ± 0.5 °C, pH; 7.9 ± 0.6, salinity; 33.1 ± 0.5‰, dissolved oxygen; 7.4 ± 0.5 mg/L). The fish were fed a commercial diet twice daily (Woosung Feed, Daejeon City, Korea). The water temperature was adjusted from ambient at a rate of ±1 °C/day until a final temperature of 24 °C was reached. The acclimation period commenced once the final temperature had been sustained for 24 h and animals were feeding while showing no sign of stress. Ammonia exposure took place in 40-L glass tanks containing 13 fish per treatment group. Ammonia chloride (NH4Cl) (Sigma, St. Louis, MO, USA) solution was dissolved in the respective glass tanks. The annual report on marine environment monitoring in Korea 2014 showed the ammonia levels 0.46 mg/L in Gunsan, 0.64 mg/L in Ulsan, and 1.39 mg/L in Busan during the summer season. By our surveying of the fish farm in Tongyeong, it showed the over 0.5 mg/L ammonia level in summer. Therefore, our studies established the experimental concentrations of ammonia 0, 0.1, 0.5, and 1.0 mg/L. The ammonia concentrations in the glass tanks were 0, 0.1, 0.5, and 1.0 mg/L, and the actual ammonia concentration is demonstrated in Table 1. The glass tank water was thoroughly exchanged once per 2 days and made the same concentration in the respective glass tank. At the end of each period (at 2 and 4 weeks), animals were anesthetized in buffered 3-aminobenzoic acid ethyl ester methane sulfonate (Sigma Chemical, St. Louis, MO).
|
Ammonia concentration (mg/L) |
||||
|---|---|---|---|---|
|
Ammonia levels |
0 |
0.1 |
0.5 |
1.0 |
|
Actual ammonia levels |
0.05 |
0.18 |
0.67 |
1.21 |
The weight and length of rockfish were measured just before exposure, at 2 and 4 weeks. Daily length gain, daily weight gain, condition factor, and Hepatosomatic Index (HIS) were calculated by the following method.
Blood samples were collected within 35–40 s through the caudal vein of the fish in 1-mL disposable heparinized syringes. The blood samples were kept at 4 °C until the blood parameters were completely studied. The total red blood cell (RBC) count, white blood cell (WBC), hemoglobin (Hb) concentration, and hematocrit (Ht) value were determined immediately. Total RBC and WBC counts were counted using an optical microscope with a hemocytometer (Improved Neubauer, Germany) after being diluted with Hendrick’s diluting solution. The Hb concentration was determined using the cyanmethemoglobin technique (Asan Pharm. co., Ltd.). The Ht value was determined by the microhematocrit centrifugation technique. Erythrocyte indices like mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were also calculated according to standard formulas.
The blood samples were centrifuged to separate serum from blood samples at 3000 g for 5 min at 4 °C. The serum samples were analyzed for inorganic substances, organic substances and enzyme activity using clinical kit (Asan Pharm. Co., Ltd.). In inorganic substances assay, calcium and magnesium were analyzed by the o-cresolphthalein complexone technique and xylidyl blue technique. In an organic substance assay, glucose and total protein were analyzed by GOD/POD technique and biuret technique. In an enzyme activity assay, glutamic oxalate transaminase (GOT) and glutamic pyruvate transaminase (GPT) were analyzed by Kind-King technique using a clinical kit.
Blood samples were collected within 35–40 s through the caudal vein of the fish in 1-mL disposable heparinized syringes. The blood samples were kept at 4 °C until the blood ammonia was completely studied. The blood ammonia was determined by indophenol method using a clinical kit (Asan Pharm. Co.,Ltd.).
The experiment was conducted in exposure periods for 4 weeks and performed triplicate. Statistical analyses were performed using the SPSS/PC+ statistical package (SPSS Inc, Chicago, IL, USA). Significant differences between groups were identified using one-way ANOVA and Duncan’s test for multiple comparisons or Student’s t test for two groups (Duncan, 1955). The significance level was set at P < 0.05.
Results
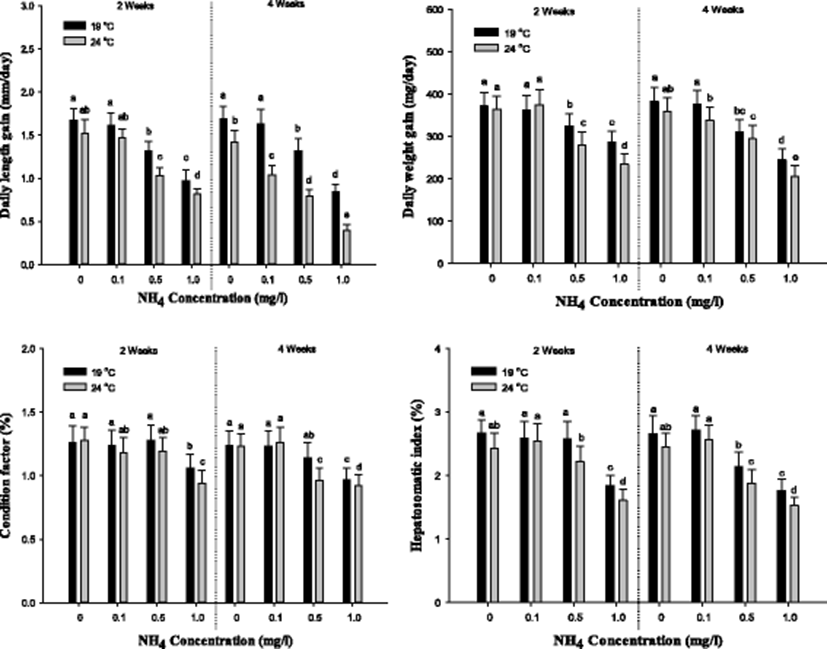
The indicators of the growth performance of S. schlegelii such as daily length gain, daily weight gain, condition factor, and hepatosomatic index are demonstrated in Fig. 1. In daily length gain, a notable reduction was observed over 0.5 mg/L at 19 and 24 °C after 2 weeks. After 4 weeks, the daily length gain was significantly decreased over 0.5 mg/L at 19 °C and over control at 24 °C. In daily weight gain, a considerable decrease was observed over 0.5 mg/L at 19 and 24 °C. After 4 weeks, the daily weight gain was markedly reduced over 0.5 mg/L at 19 °C and over 0.1 mg/L at 24 °C. The condition factor after 2 weeks was substantially decreased in the concentration of 1.0 mg/L at 19 and 24 °C, and a considerable decrease after 4 weeks was observed in the concentration in 1.0 mg/L at 19 °C and over 0.5 mg/L at 24 °C. In the Hepatosomatic Index, a significant reduction was observed in the concentration of 1.0 mg/L at 19 °C and over 0.5 mg/L at 24 °C. After 4 weeks, the Hepatosomatic Index was notably decreased over 0.5 mg/L at 19 and 24 °C. In the growth performance, the growth indicators affected the concentration of ammonia exposure and temperature.
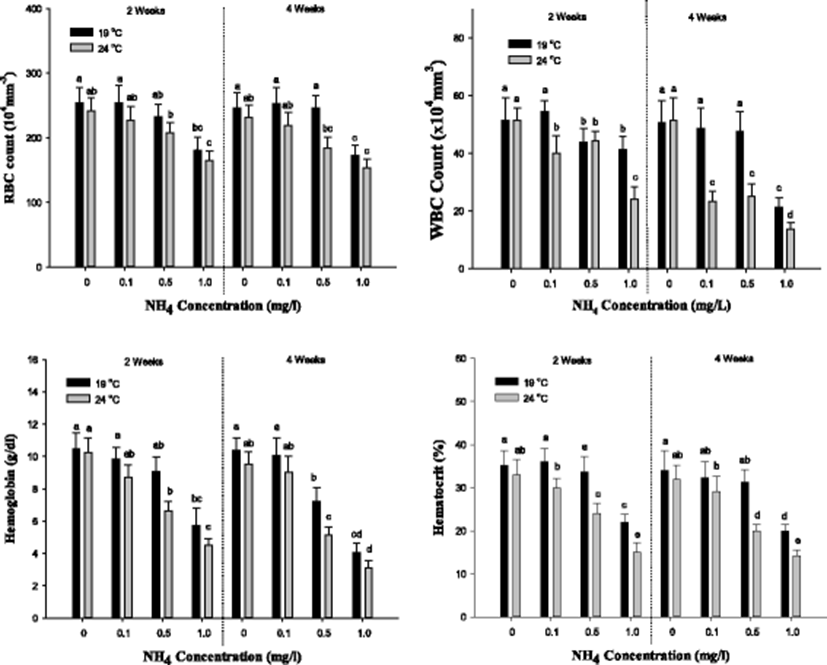
The hematological parameters (RBC count, WBC count, Hb, and Ht; MCV, MCH, and MCHC) of S. schlegelii is demonstrated in Figs. 2 and 3. In RBC count, a notable decline was shown in the concentration of 1.0 mg/L at 19 °C and over 0.5 mg/L at 24 °C after both 2 and 4 weeks. In WBC count, a substantial decrease was observed over 0.5 mg/L at 19 °C and over 0.1 mg/L at 24 °C after 2 weeks. After 4 weeks, the WBC count was markedly increased in the concentration of 1.0 mg/L at 19 °C and over 0.1 mg/L at 24 °C. In hemoglobin, a significant decrease was observed in the concentration of 1.0 mg/L at 19 °C and over 0.5 mg/L at 24 °C after 2 weeks. After 4 weeks, the Hb concentration was decreased over 0.5 mg/L of ammonia exposure. In hematocrit, a considerable decrease was observed in the concentration of 1.0 mg/L at 19 °C and over 0.5 mg/L at 24 °C after both 2 and 4 weeks. The values of RBC count, WBC count, Hb, and Ht were notably decreased by the ammonia exposure, and the high temperature catalyzed the decline in the values.
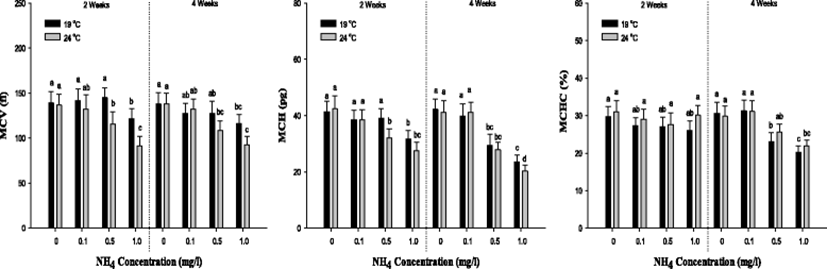
In MCV value, a significant decrease was observed in the concentration of 1.0 mg/L at 19 °C and over 0.5 mg/L 24 °C after 2 and 4 weeks. The MCH value was considerably decreased in the concentration of 1.0 mg/L at 19 °C and over 0.5 mg/L 24 °C after 2 and 4 weeks. In MCHC value, there was no notable alteration after 2 weeks. But, a substantial decline was observed over 0.5 mg/L of ammonia exposure after 4 weeks. The values of MCV, MCH and MCHC were markedly reduced by the high ammonia exposure and temperature.
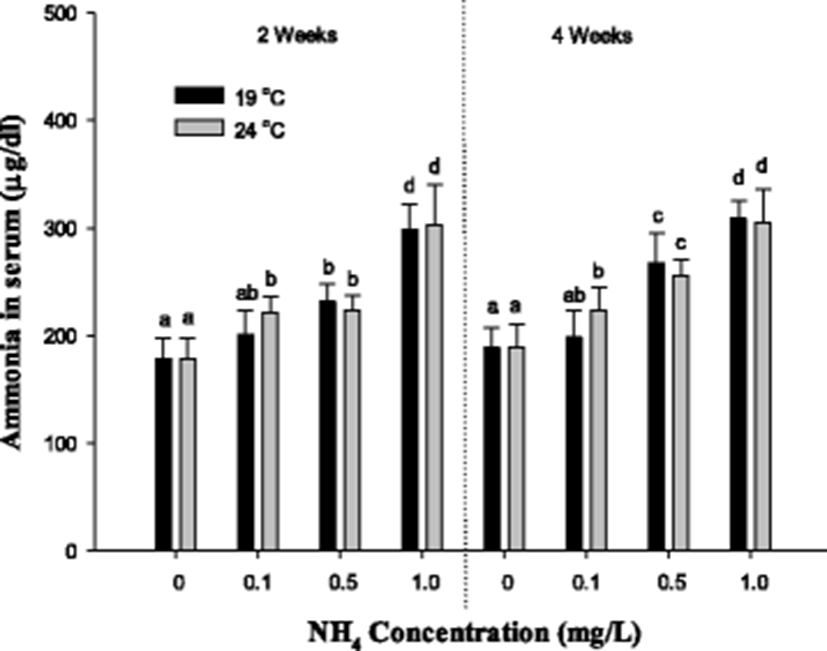
The blood ammonia concentration of S. schlegelii is shown in Fig. 4. The blood ammonia concentration of S. schlegelii was considerably increased over 0.5 mg/L at 19 °C and 0.1 mg/L at 24 °C after 2 and 4 weeks. There was no notable alteration according to the difference of temperature except for the concentration of 0.1 mg/L of ammonia exposure.
The serum inorganic components such as calcium and magnesium of S. schlegelii are shown in Table 2. There was no alteration in calcium and magnesium of S. schlegeliiby ammonia exposure depending on temperature. The serum organic components such as total protein and glucose of S. schlegelii are demonstrated in Table 3. In total protein, a notable decrease was observed in the concentration of 1.0 mg/L at 19 °C and over 0.5 mg/L at 24 °C. The total protein was considerably decreased over 0.5 mg/L at 19 and 24 °C after 4 weeks. In glucose, a substantial increase was observed in the concentration of 1.0 mg/L at 19 °C and over 0.5 mg/L at 24 °C. The glucose was substantially reduced over 0.5 mg/L at 19 and 24 °C after 4 weeks. The serum enzyme components such as GOT and GPT of S. schlegelii are shown in Table 4. The GOT was significantly increased over 0.5 mg/L at 19 and 24 °C after 2 and 4 weeks. In GPT after 2 weeks, a notable increase was observed over 0.5 mg/L at 24 °C, whereas there was no change at 19 °C. After 4 weeks, the GPT was considerably increased in the concentration of 1.0 mg/L at 19 °C and over 0.5 mg/L at 24 °C.
Discussion
The toxicant exposure can induce the inhibition of growth performance in aquatic animals. Erickson et al. (2010) reported a significant reduction of growth performance of rainbow trout, Oncorhynchus mykiss, exposed to arsenic. In this study, the ammonia exposure caused a notable decrease in growth performance of S. schlegelii. And, the reduction of growth performance may result from the demand for energy to detoxicate the ammonia which effects the drop in the energy for growth (Clearwater et al. 2002). Also, the growth performance of S. schlegelii was affected by the water temperature change. Carvalho and Fernandes (2006) reported that the high temperature causes the severe accumulation in fish by the toxicant exposure, which may need the more energy for detoxification.
The biochemical and physiological alterations in fish blood can be occurred by the toxic substances in an aquatic environment, and the blood parameters can be a sensitive and reliable indicator to evaluate the physiological status of fish (Mazon et al. 2002. Vosyliene and Kazlauskiene (2004) reported a negative change in blood chemistry of rainbow trout, O. mykiss, exposed to ammonia. In this study, the RBC count, WBC count, hemoglobin, and hematocrit of S. schlegelii were substantially decreased by ammonia exposure. Tilak et al. (2007) also reported a substantial decrease in hemoglobin of common carp, Cyprinus carpio, exposed to ammonia, which is caused by the increase in the oxygen intake and elevation in methemoglobin by gill damage. Thangam et al. (2014) reported a notable reduction in RBC and WBC count of common carp, C. carpio, exposed to ammonia. RBC count decreased due to anemia leading to inhibition of erythropoietin, and WBC count also decreased by the leucopenia coupled with stress for toxicants in aquatic animals. Knoph and Thorud (1996) reported that Atlantic salmon, Salmo salar, when exposed to ammonia showed decreased hematocrit resulting from reduction in RBC count. The ammonia exposure also caused a notable reduction in MCV, MCH, and MCHC of S. schlegelii. Saravanan et al. (2011) suggested that the diazinon pesticide exposure to European catfish, Cyprinus carpio resulted in a considerable decrease in MCV, MCH, and MCHC, which is due to the increase of immature red blood cells by the toxicant exposure. In this study, the temperature in addition to the ammonia concentration substantially affected the hematological parameters of S. schlegelii. Adeyemo et al. (2003) reported that temperature decreases both the quantity and quality of erythrocytes and hemoglobin, which cause deteriorated oxygen supply. Carvalho and Fernandes (2006) suggested that a high temperature considerably affected the hematological values of Prochilodus scrofa exposed to copper result in the increased diffusion rate, chemical reactions, and increased oxygen transport at a high temperature.
The ammonia exposure induced a significant accumulation in the blood of S. schlegelii. Lemarie et al. (2004) also reported a considerable ammonia accumulation in the blood of juvenile sea bass, Dicentrarchus labrax, exposed to ammonia, which should negatively affect the experimental animal. The ammonia concentration notably affected the ammonia accumulation in the blood of S. schlegelii, but there was no significant change in the ammonia accumulation in the blood according to the temperature.
The inorganic serum components such as calcium and magnesium have been considered as critical indicators to assess the toxicity of substances, which also act as the ion regulator for homeostasis (Kim and Kang 2015). In inorganic serum components, there was no alteration in calcium and magnesium of S. schlegelii exposed ammonia. Knoph and Thorud (1996) also reported no significant change in calcium and magnesium of Atlantic salmon, S. salar, exposed to ammonia for 2 weeks, whereas a notable increase in calcium and magnesium was observed after 2–3 days of exposure time. Thus, our studies showed that exposure at 2 and 4 weeks was not influenced in plasma, calcium, and magnesium concentrations. The organic components such as total protein and glucose can be a reliable biomarker to detect animal health (Oner et al. 2007). In organic serum components, the total protein of S. schlegelii was significantly decreased by ammonia exposure, whereas the glucose of S. schlegelii was increased. Gopal et al. (1997) suggested that total plasma has been notably changed under stress situations. The increase in glucose may be a consequence of glycogenolytic activity of catecholamines and gluconeogenetic effect of glucocorticoids by the stress response under toxic substance exposure (Dobsikova et al. 2011). The GOT and GPT in serum components can be generally used to assess the tissue damage of the liver and kidney (Agrahari et al. 2007). In enzyme serum components, the GOT and GPT of S. schlegelii was significantly increased by the ammonia exposure. Vedel et al. (1998) also reported a considerable increase in the GOT and GPT of rainbow trout, O. mykiss, exposed to ammonia, indicated some degree of tissue necrosis. The temperature as well as the concentration of ammonia exposure notably affected the alterations of serum components of S. schlegelii, showing that the temperature also can be a critical factor to affect the experimental animals.
Conclusions
The ammonia exposure to S. schlegelii depending on water temperature induced notable decreases in growth performance (daily length gain, daily weight gain, condition factor, and hepatosomatic index) hematological parameters (RBC count, WBC count, hemoglobin, and hematocrit) and significant alterations in serum components (total protein, glucose, GOT, and GPT). Considering the results of this study, the ammonia depending on water temperature should negatively influence the experimental fish, S. schlegelii.