Background
Within the last 30 years, seaweed cultivation has attained large-scale production levels and has become a major industry in China, Korea, and Japan. Korea had a harvest of 1,022,326 tons in 2012, which makes it the fourth largest seaweed-producing country in the world (FAO, 2014). The economically important seaweed species harvested in Korea are laver Porphyra spp., wakame Undaria spp., kelp Laminaria spp., fusiforme, Hizikia fusiformis, and green laver Monostroma spp. (KOSIS, 2015).
Kelp Saccharina japonica cultivation was industrialized in Korea in the 1970s, and from 2005, it has been extensively developed together with the abalone industry. During the 1970s to 1980s, kelp production reached less than 12,000 tons, and production steadily increased since then with about 370,000 tons harvested in 2013 (KOSIS, 2015). Kelp production now accounts for about 25% of all Korean seaweed cultivation (KOSIS, 2015). However, the increase in production has also resulted in kelp farms becoming severely infected by several epiphytic organisms (Gong et al., 2010; Park and Hwang, 2012). An infestation is characterized by the appearance of numerous colonies on the thallus of the kelp, and therefore, the affected kelp appears unsightly and cannot be sold. Although systematic research on fish disease has been conducted in Korea since the 1990s, seaweed disease has not yet been systematically studied. Therefore, little information is available on kelp disease. The present study consists of a survey that was conducted to investigate the presence of epiphytic organisms in Korean kelp farms. In addition, a histopathological examination was conducted to understand the effect by epiphytic organisms on the thallus.
Methods
Kelp samples were obtained from four private farms in Wando (Southern Sea), Busan (Southern Sea), and Pohang (Eastern Sea) during 2014 and 2015 (Table 1, Fig. 1). Wando is the most popular area for kelp cultivation, producing about 97% of the kelp crop of Korea. The kelps were randomly selected by a farmer, transported on ice, and immediately subjected an examination of the epiphytic organisms. The total length (from apex to holdfast) of the thallus was measured, and the surfaces of the specimens were examined macroscopically and microscopically for the presence of epiphytic organisms. The detection rate of the epiphytic organisms was measured by calculating the percentages of the kelp infested by epiphytic organisms, and their infestation area was calculated as the percentage of the thallus that had been colonized. The number of caprellid was measured in a 2 × 2 cm kelp area infected with hydroid because their infestation area cannot be calculated. The epiphytic organisms that were obtained were photographed and identified according to their morphological characteristics (Gong et al., 2010; Kim and Lee, 1975; Lee, 1994; Park, 2010; 2011; Saunders and Metaxas, 2009; Schwaninger, 1999; The Korean Society of Systematic Zoology, 2014).
NT not tested
Results
Table 1 shows the results of the detection of epiphytic organisms. Of the 740 kelp samples, 208 had six kinds of epiphytic organisms attached, including hydroid, bryozoan, polychaete, algae, caprellid, and oyster (Fig. 2). Of these, hydroid, bryozoan, polychaete, algae, and caprellid were frequently observed and were detected in the kelp samples with rates of 11.6% (86/740), 6.4% (47/740), 3.4% (25/740), 3.2% (24/740), and 3% (22/740), respectively.

Two hundred thirteen samples were examined in the Wando A farm (Table 1). Hydroid, bryozoan, algae, caprellid, and oyster were detected in 7.5% (16/213), 5.6% (12/213), 5.6% (12/213), 2.8% (6/213), and 0.9% (4/213) kelp, respectively, and these organisms were only observed during the period from June to September in 2014. Twenty to 100% were found to be hydroid-positive, 50 to 71.4% were bryozoan-positive, and 14.3 to 100% were algae-positive. Twenty and 71.4% of the caprellid were observed on hydroids, but not on kelp. The oyster (57.1%) was observed in the September 2014 sample. Epiphytic organisms were not observed among the kelp during April to May in 2014 and January to April in 2015. The infestation areas for hydroid, bryozoan, and algae were 10 to 85%, 0.3 to 17%, and 20 to 30%, respectively (Table 2). Two and eight caprellids were observed in the June sample and in the August sample of 2014, respectively.
NT not tested
aMean number of caprellid
Two hundred fifty-three samples were examined in the Wando B farm (Table 1). Hydroid, bryozoan, polychaete, algae, and caprellid were detected in 13% (33/253), 2% (5/253), 2% (5/253), 1.6% (4/253), and 0.4% (1/253) kelp, respectively, and these organisms were observed from June to September in 2014. 57.1 to 100% were found to be hydroid-positive, and 33.3, 35.7, and 13.3 to 28.6% were bryozoan-positive, polychaete-positive, and algae-positive, respectively. The caprellid (7.1%) was observed on the hydroids in the July 2014 sample. No epiphytic organisms were observed in the kelp in May 2014 and from February to April 2015. The infestation areas for hydroid gradually increased from 10 to 55% as the water temperature increased, but these were lower than those of the Wando A farm (Table 2). The infestation areas for bryozoan, polychaete, and algae were observed in 0.13% (0.06–0.5%) in the September sample, 3% (1–5%) in the July sample, and 5% (3–6%) in the June sample, 2014, respectively. Two caprellids were observed in the July 2014 sample.
Two hundred thirty-six samples were examined in the Busan farm (Table 1). Hydroid, bryozoan, algae, and caprellid were detected in kelp with an incidence rate of 11% (26/236), 9.7% (23/236), 3.4% (8/236), and 4.2% (10/236), respectively, and these organisms were observed from April to July 2014. One hundred percent of the samples were found to be hydroid-positive from May to July 2014. Ten to 90% and 30 to 71.4% were bryozoan-positive and algae-positive, respectively. No epiphytic organisms were observed among the kelp from February to April 2015. The infestation areas of the hydroid were 14.4 to 37%, which are lower than those of the two farms in Wando (Table 2). In contrast, the infestation areas for bryozoan were of 0.04 to 37.9%, which were the highest in the four farms. 34.1% of the algae were observed in June 2014, and three caprellids were observed in July 2014.
In the Pohang farm, hydroid, bryozoan, and polychaete were detected in kelp with rates of 28.9% (11/38), 18.4% (7/38), and 52.6% (20/38), respectively, from July to September 2014 (Table 1). The infestation areas for hydroid and bryozoan were less than 5.5%, which were the lowest in the four farms (Table 2). In contrast, the infestation areas for polychaete were 9.5 to 90%, which were the highest of the four farms.
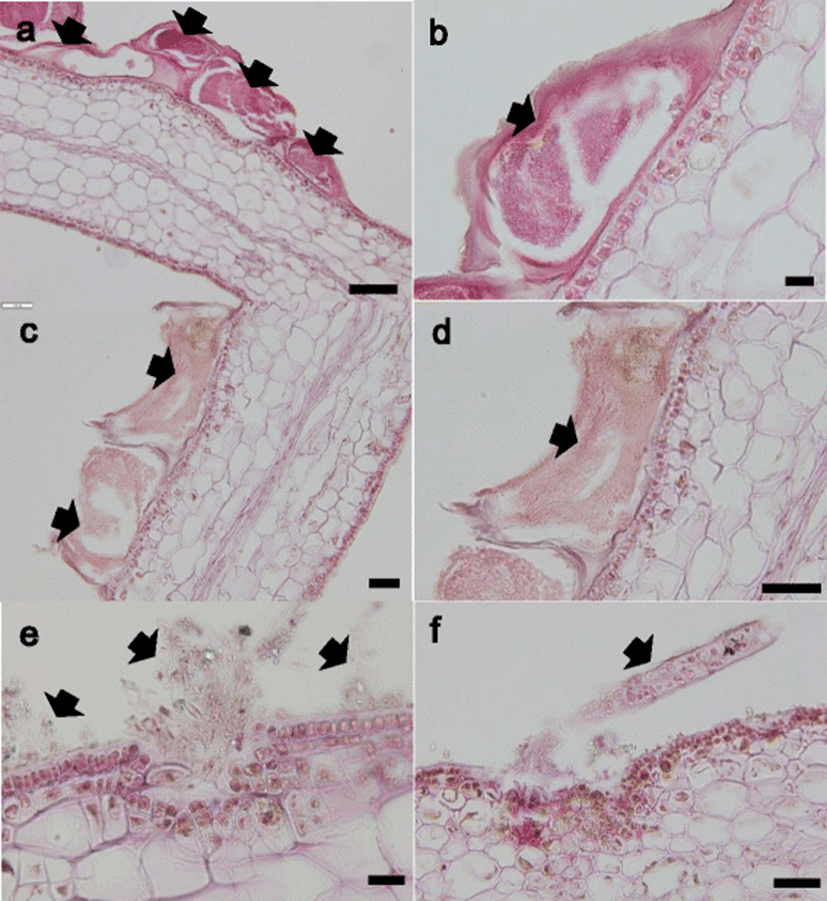
The hydroid, bryozoan, and algae that were attached to the thallus were examined (Fig. 3). The hydroid and bryozoan were attached to the cuticula of the thallus but did not penetrate into the thallus (Fig. 3a–d). In contrast, some of the algae penetrated the epidermis and attached to the cuticula of thallus (Fig. 3e, f).
Discussion
Korean farms have been cultivating kelp since the 1970s, but to date, no systematic research on their disease has been conducted. In this study, we investigated the presence of epiphytic organisms in four kelp farms in the coastal area of Korea. Of the 740 kelp samples, 208 samples had epiphytic organisms attached, including hydroid, bryozoan, polychaete, algae, caprellid, and oyster. The predominant epiphytic organisms were hydroid (detection rate: 11.6%) followed by bryozoan (6.4%), polychaete (3.4%), algae (3.2%), and caprellid (3%), as observed from May to September. No epiphytic organisms were observed among the kelp from January to April, except for one sample from Busan. These results indicate that at least six kinds of epiphytic organisms were observed in kelp farms in Korea, and their infestation was significantly higher in water with a higher temperature. Moreover, encrusting hydroid and bryozoan were the most predominant form of infestation in kelp farms, even though their infestation rates were different among the kelp farms. Encrusting by hydroid has been reported to be abundant on farmed kelp in Korea at higher water temperatures (Park and Hwang, 2012). The infestation rate from May to July was of about 97%, and it was below 26% from February to April. These results are similar to the results of the present study. Encrusting by bryozoan (Membranipora) has been reported to be abundant on kelp blades in Atlantic Nova Scotia (Canada) and the Gulf of Maine (USA) (Berman et al., 1992; Saunders and Metaxas, 2008; Scheibling and Gagnon, 2009). The calcified Membranipora zooids present a firm mechanical barrier on epidermal tissue. This barrier can affect exchange processes such as mineral nutrient uptake between kelp epidermis and surrounding seawater (Hurd et al., 1994; 2000), or it can interfere with the photophysiology of the host alga (Oswald et al., 1984; Cancino et al., 1987; Muñoz et al., 1991). In this study, even though the effect of the bryozoan on kelp is unknown, Korean kelp farm is suffered from infestation by bryozoan.
It is unclear how the epiphytic organisms attach to the thallus tissue. A histopathogical examination revealed that hydroid and bryozoan were attached on the cuticula of thallus while some algae attached to the cuticula of the thallus or penetrated the epidermis. These results indicate that kelp tissue can be broken by some algae, but not by hydroid and bryozoan.
The cultivation period for kelp in Korea had traditionally spanned from December to July, with a harvest of thalli occurring between June and July for use with edible marine vegetable food. Recently, the harvest season has been shortened from March to May due to the presence of these epiphytic organisms. Moreover, kelp that is severely infected by hydroid and bryozoan is used as abalone feed during the summer, even though their effect on abalone remains. In addition, there are many environmental factors that can affect the prevalence of the epiphytes on kelps. Therefore, further studies are necessary to elucidate which environmental factors are related to the epiphytic organisms’ infestation, to understand how to prevent the epiphytic organisms from infesting kelp farms and to assess their effect when used as abalone feed.
Conclusion
In conclusion, we investigated the presence of epiphytic organisms in four kelp Saccharina japonica farms in the coastal area of Korea from 2014 to 2015. The infestation rate for hydroid, bryozoan, and polychaete was significantly higher in the Wando farm, Busan farm, and Pohang farm, respectively. Epiphytic organisms were generally observed during May to September. The histopathogical examination revealed that hydroid and bryozoan organisms were attached on the cuticula of the thallus. These results indicate that hydroid and bryozoan were the most predominant epiphytic organisms in Korean kelp farms.