Background
Taurine (2-aminoethane sulfonic acid) is an amino acid synthesized from methionine via cysteine by a series of enzymatic reactions (Oja and Kontro 1983). It is considered as a conditionally indispensable amino acid for marine or cold water fish species. The ability of fish to synthesize taurine is species or developmental stage dependent (Yokoyama et al. 2001; Kim et al. 2003, 2005). The reason for such differences in their ability to synthesize taurine might be due to various activities of L-cysteine sulfinate decarboxylase, an enzyme required for the conversion from cysteine to taurine (De la Rosa and Stipanuk 1985; Takeuchi et al. 2001; Yokoyama et al. 2001). Although taurine is nonessential, its inclusion in diets is often recommended because of its auxiliary actions such as membrane protection, anti-oxidation and detoxification in mammals (Wright et al. 1986). In addition, it plays a role in osmoregulation in invertebrates (Schaffer et al. 2000), acts as a carrier for lipid-soluble vitamins in mammals (Petrosian and Haroutounian 2000) and induces bile salt production in fish (Van Waarde 1988). Due to its unique amino acid properties (i.e. low molecular weight, nitrogen content and water solubility), taurine can also act as a feed stimulant for fish (Carr 1982). It has been reported that taurine is effective as a feed attractant by stimulating the olfactory system of arctic char (Salvelinus alpinus), grayling (Thymallus thymallus) (Doving et al. 1980) and rainbow trout (Oncorhynchus mykiss) (Hara et al. 1984).
Effects of water temperature on nutrient utilization have been studied in many fish species (Olsen and Ringn 1998; Peres and Oliva-Teles 1999). It has been reported that the optimum temperature for growth of Japanese flounder is 20–25 °C, even though they can be fed at low temperatures ranging from 10 to 20 °C (Iwata et al. 1994). In most studies, nutrient requirements for flounder have been determined at moderate water temperatures (18–22 °C) (Lee et al. 2000, 2002).
Olive flounder is the most important marine fish species in Korea with production exceeding 60% of annual fish production (Ministry of Maritime Affairs and Fisheries 2015). Olive flounder can be exposed to suboptimal temperatures during the culture period in Korea. Thus, knowledge on nutrient utilization in different water temperatures would be useful in optimizing dietary compositions or feeding conditions throughout the year for the growth of olive flounder. However, limited information is available on nutrient utilization in suboptimal water temperatures for this species. Therefore, the objective of this study was to determine the effects of taurine supplementation on growth, feed utilization, whole-body composition, innate immunity, and liver mRNA expression of IGF-1 of juvenile olive flounder at low water temperatures.
Methods
Five experimental diets (Table 1) were formulated to contain taurine in fish meal-based diets at 0, 0.25, 0.5, 1.0, and 1.5% (Control, T1, T2, T3, and T4) for juvenile olive flounder. The five diets were formulated to be isonitrogenous (49.0% crude protein) and isocaloric (18.1 kcal/kg diet). All dry ingredients were thoroughly mixed with 10–15% double distilled water after addition of fish oil, pelleted through a pellet machine (SP-50, Gumgang Engineering, Daegu, Korea) at 6 mm in diameter, dried for 24 h and stored at −20 °C until used.
aMineral premix (g/kg mixture): MgSO4.7H2O, 80.0; NaH2PO4.2H2O, 370.0; KCl, 130.0; Ferric citrate, 40.0; ZnSO4.7H2O, 20.0; Ca-lactate, 356.5; CuCl2, 0.2; AlCl3. 6H2O, 0.15; Na2Se2O3, 0.01; MnSO4.H2O, 2.0; CoCl2.6H2O, 1.0
bVitamin premix (g/kg mixture): L-ascorbic acid, 121.2; DL-a tocopheryl acetate, 18.8; thiamin hydrochloride, 2.7; riboflavin, 9.1; pyridoxine hydrochloride, 1.8; niacin, 36.4; Ca-D-pantothenate, 12.7; myo-inositol, 181.8;D-biotin, 0.27; folic acid, 0.68; p-aminobezoic acid, 18.2; menadione, 1.8; retinyl acetate, 0.73; cholecalficerol, 0.003; cyanocobalamin, 0.003
Olive flounder juveniles were purchased from a private hatchery and transported to Marine and environmental Research Institute, Jeju National University, Jeju, South Korea. Fish were adapted to experimental conditions and facilities for 2 weeks. At the end of the acclimation period, total 525 fish (initial mean body weight, 19.5 g) were randomly distributed into 15 150 L capacity polyvinyl circular tanks with three replicate groups per diet treatment. The tanks were supplied with filtered seawater at a flow-rate of 3 L/min and aeration to maintain enough dissolved oxygen. Each tank was designated as one of three replicates for a diet group. They were fed with the experimental diets to apparent satiation two times a day (08:30 and 17:00 h) for 10 weeks. Water temperature was naturally maintained at 16.4 ± 0.36 °C during the whole duration. Growth measurement was carried out at every 2 weeks.
At the end of the feeding trial, all the fish in each tank were bulk-weighed and counted for calculation of weight gain (WG), specific growth rate (SGR), feed conversion ratio (FCR), protein efficiency ratio (PER), and survival. Four fish per tank (4 fish/tank, 12 fish/diet) were randomly captured 24 h after the last meal, anesthetized with 2-phenoxyethanol solution (200 mg/L), and blood samples were taken from the caudal vein with heparinized syringe for determination of hematocrit, hemoglobin, and nitro-blue-tetrazolium (NBT) activity. Then, plasma samples were separated by centrifugation at 5000×g for 10 min and stored at −70 °C for determination of plasma chemicals and total immunoglobulin (Ig) level. Another set of blood samples (4 fish/tank, 12 fish/diet) were taken using non-heparinized syringe and allowed to clot at room temperature for 30 min. The serum was separated by centrifugation for 10 min at 5000×g and stored at −70 °C for the analysis of non-specific immune responses. Three intact fish per tank (3 fish/tank, 9 fish/diet) were randomly selected and kept at −70 °C for whole-body composition analysis. Hematocrit was determined in four individual fish per tank by a microhematocrit method (Brown 1980). Hemoglobin, glucose, total protein and total cholesterol were determined in the same four fish by using the automated blood analyzer (SLIM, SEAC Inc., Florence, Italy). Oxidative radical production by phagocytes during respiratory burst was measured through the NBT activity assay described by Anderson and Siwicki (1995). Serum myeloperoxidase (MPO) activity was measured by Quade and Roth (1997). Superoxide dismutase (SOD) activity was measured by percentage reaction inhibition rate of enzyme with water soluble tetrazolium dye substrate and xanthine oxidase using a SOD assay kit (Sigma- aldrich, 19,160, St. Louis, USA) according to the manufacturer’s instructions. Plasma total Ig level was determined by Siwicki and Anderson (1993). Serum lysozyme level was measured using turbidometric assay described by Hultmark et al. (1980). The serum anti-protease activity was measured by Ellis (1990) with slight modifications (Magnadottir et al. 1999). Moisture and ash content were analyzed by the standard procedures (AOAC 2005). Crude protein were measured by using automatic Kjeltec Analyzer Unit 2300 (FOSS, Hillerød, Sweden) and crude lipid was analyzed by Folch et al. (1957). Amino acid composition of the diets was determined using a Sycom S-433D automatic amino acid analyzer (Sykam, Eresing, Germany). Hydrolysis of the samples was performed in 6 N HCl at 110 °C for 24 h under nitrogen atmosphere. Identification and quantification of each amino acid were achieved by comparing the retention times of the peaks with those of standards.
Liver samples were taken from two fish per tank and total RNA was isolated using trizol reagent following the manufacturers’ protocol. The quantity of the RNA was calculated using the absorbance at 260 nm. The integrity and relative quantity of RNA were checked by gel electrophoresis. PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa Code DRR047) was used to remove genomic DNA and reverse transcription. Levels of IGF-1 transcript were measured by real-time PCR (SYBR Green I), using 18S rRNA as a housekeeping gene. Primers for real-time PCR were designed based on the previously cloned sequence for IGF-1 (NCBI Genbank accession no: AF061278) and 18S rRNA (NCBI Genbank accession no: EF126037) in P. olivaceus. Relative expression ratio of IGF-1 was calculated based on the PCR efficiency (E) and the Ct of a sample versus the control (FM treatment) and expressed in comparison with the reference gene (18S rRNA); according to Pfaffl’s mathematical model (Pfaffl 2001).
The diets were assigned by a completely randomized design. Data were analyzed by one-way analysis of variance (ANOVA) in SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). When ANOVA identifies differences among groups, the difference in means was made with Tukey’s HSD multiple range test. Statistical significance was determined at P < 0.05 for means of treatments. Percentage data were arcsine transformed before statistical analysis.
Results
Growth performance and feed utilization of juvenile olive flounders are shown in Table 2. All the taurine-supplemented groups showed numerically increased growth compared to the control even though it was not significant. The highest growth rate was found in fish fed T3 diet. However, feed utilization was significantly influenced by the taurine supplementation reducing FCR and increasing PER. FCR was significantly lower in fish fed all the taurine-supplemented diets than that of fish fed the control diet. Higher supplementation (T3 and T4) of taurine exhibited even significantly lower FCR than that of fish fed T1 and T2 diets. PER was significantly higher in fish fed T2, T3, and T4 diets compared to that of fish fed the control and T1 diets.
Values are mean of triplicate groups and presented as mean ± S.D. Values with different superscripts in the same row are significantly different (P < 0.05). The lack of superscript letter indicates no significant differences among treatments
1Final body weight (g)
2Weight gain (%) = 100 × (final mean body weight – initial mean body weight)/initial mean body weight
3Specific growth rate (%/day) = [(ln final body weight – ln initial body weight)/days] × 100
4Feed intake (g fish−1) = dry feed consumed/fish number
5Feed conversion ratio = dry feed fed/wet weight gain
6Protein efficiency ratio = wet weight gain/total protein given
Hematological responses of fish fed the diets are shown in Table 3. Hematocrit and hemoglobin levels were significantly elevated by taurine supplementation. T3 group had the highest hematocrit and hemoglobin levels among all the groups. Total cholesterol level was significantly decreased in T4 group compared to the control group.
Values are mean of triplicate groups and presented as mean ± S.D. Values with different superscripts in the same row are significantly different (P < 0.05). The lack of superscript letter indicates no significant differences among treatments
1Hematocrit (%)
2Hemoglobin (g dL−1)
3Glucose (mg dL−1)
4Total protein (g dL−1)
5Total cholesterol (mg dL−1)
Results of non-specific immune response of fish fed the diets are shown in Table 4. All the immune parameters showed promising results in fish fed taurine-supplemented diets. All the taurine supplementation groups showed significantly higher NBT, MPO, Ig, and antiprotease activities compared to the control group. In SOD and lysozyme activities, significantly higher values were observed in taurine supplementation over 0.5% in diets except for T4 group. The highest NBT and lysozyme activities were shown in T3 group while the highest MPO, SOD, and antiprotease activities were in T2 and T3 groups. The highest Ig level was observed in T2 group.
Values are mean of triplicate groups and presented as mean ± S.D. Values with different superscripts in the same row are significantly different (P < 0.05). The lack of superscript letter indicates no significant differences among treatments
1Nitro blue tetrazolium activity
2Myeloperoxidase level
3Superoxide dismutase (% inhibition)
4Total immunoglobulin (mg mL−1)
5Lysozyme activity (μg mL−1)
6Antiprotease (% inhibition)
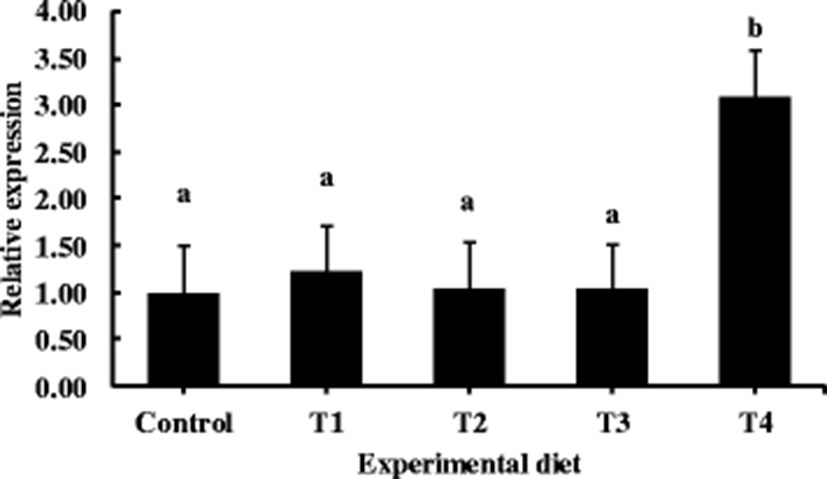
Results of proximate compositions in whole-body samples are shown in Table 5. No significant effects were observed by the supplementation of taurine. Relative expression levels of IGF-1 mRNA is shown in Fig. 1. There was not much of an influence on expression, however, T4 group showed a significantly higher peak compared to other groups including the control group.
Values are mean of triplicate groups and presented as mean ± S.D. Values with different superscripts in the same row are significantly different (P < 0.05). The lack of superscript letter indicates no significant differences among treatments
Discussion
Increased feed intake is a primary response of fish to increased water temperature (Bureau et al. 2002). Improved feed intake at high water temperature generally results in higher growth and feed efficiency (Kim et al. 2006). According to this phenomenon, reduced growth of fish in cold water is due to reduced feed intake. It was reported that fish fed an extruded feed at suboptimal water temperatures had relatively lower growth performance than that of fish fed raw fish or Oregon moist pellet (Satoh et al. 2003). This phenomenon is called winter syndrome. To solve the problem of winter syndrome, it is important to improve feed intake or feed utilization to obtain better growth performance at low water temperature. Therefore, the present study was conducted to determine whether taurine supplement could improve feed intake of olive flounder at low water temperature.
It has been reported that taurine supplementation can improve growth performance of many fish species including olive flounder, parrot fish (Oplegnathus fasciatus), red seabream (Pagrus major) and yellow tail (Seriola quinqueradiata) (Kim et al. 2007; Matsunari et al. 2008; Takagi et al. 2008; Lim et al. 2013; Takagi et al. 2013; Hanini et al. 2013; Han et al. 2014; Khaoian et al. 2014). Kim et al. (2007) reported that the taurine requirement of olive flounder is 1.0% for juvenile and fingerling stages in optimal water temperature. Han et al. (2014) reported that taurine and glutamine supplementations can significantly improve growth, feed intake and FCR of olive flounder at optimal water temperature. However, studies on the effect of taurine supplementation at suboptimal water temperature are very limited. Water temperature varies depending on seasons for many regions. Fish shows normal behavior and best growth performance only in optimal water temperature because fish is a poikilotherm. In suboptimal water temperature, feed intake of fish usually decreases resulting in decreased growth performance. To solve the problem of winter syndrome, various methods using several feed stuffs such as enzyme treated fish meal, krill extract, or krill meal have been evaluated (Satoh 2003; Satoh et al. 2003). Satoh et al. (2003) have reported that daily growth rate and apparent protein digestibility of fish-fed diet with protease are worse than those fed with MP at low water temperature. However, there is no significant difference in feed efficiency between fish fed with diet treated with protease and those fed with MP diets (dry matter basis).
In the present study, growth of juvenile olive flounder was numerically increased by taurine supplementation resulting in improved FCR and PER, because feed intake was not different among all the groups. Therefore, feed efficiency was positively influenced by taurine supplementation. Chatzifotis et al. (2008) reported that in a cold water condition, similar tendency to the current results was observed in common dentex (Dentex dentex)-fed diet with taurine supplementation. Cook et al. (2003) investigated on winter syndrome of snapper (Lutjanus campechanus) and found similar results by dietary supplementation of β-glucan. Several studies in different fish species have reported similar results. Park et al. (2002) and Kim et al. (2007) reported that dietary taurine can significantly enhance the growth performance of juvenile Japanese flounder. The observations are similar to the results of juvenile yellowtail (Matsunari et al. 2005) and turbot (Scophthalmus maximus) (Qi et al. 2012). Growth rate and feed efficiency have been improved with taurine supplementation in European sea bass (Dicentrarchus labrax) (Brotons Martinez et al. 2004), rainbow trout (Gaylord et al. 2006), and black tiger shrimp (Penaeus monodon) (Shiau and Chou 1994).
Hematological parameters are also improved by the taurine supplementation. Blood parameters indicate the healthiness of fishes (Lemaire et al. 1991; Kader et al. 2010). Results of the present study showed that hematocrit and hemoglobin levels were significantly increased by taurine supplement. Similar results have been reported by Han et al. (2014) for olive flounder. High levels of cholesterol often indicate physiological disorders of fish (Eslamloo et al. 2012). In the present study, plasma cholesterol levels were high in fish fed the control diet. However, they were significantly reduced by taurine supplement. This might indicate that the fish fed the control diet were relatively under stresses. However, supplemental taurine feeding helped the fish recover at some degree, although such effect was not shown in survival rate. Non-specific immune response was the other major aspect we considered. Since the fish were in high stress conditions or challenging environment like in the suboptimal water temperature, if there was any effect of the dietary supplementation it could appear. Results of this study revealed that all the innate immune parameters of olive flounder could apparently be enhanced by dietary taurine supplement especially in a low water temperature condition. Fish in all groups fed taurine showed significantly higher innate immune responses than fish in the control group. Many studies have determined if taurine has positive effect on immunity of fish. Fang et al. (2002) reported that taurine can directly scavenge free radicals. Higuchi et al. (2012) also showed elevated SOD levels in eels fed a taurine supplemented diet. Similarly, Han et al. (2014) reported higher SOD levels in olive flounder fed diets supplemented with taurine. Interestingly, the present study also showed that SOD and MPO levels were significantly increased in fish fed diets with taurine supplementation. Lysozyme is an important defense enzyme of innate immune system of fishes (Saurabh and Sahoo 2008) and has been used as a key parameter to evaluate non-specific defense ability (Zhou et al. 2006; Ren et al. 2007). Anti-protease is an enzyme which disables enzymes generated by a pathogen inside an organism (Magnadottir et al. 1999). Therefore, an increased level of anti-protease indicates an increase in immunity. In the present study, taurine-fed groups showed significant increment in both lysozyme and anti-protease activities. Similarly, Li et al. (2016) observed significant increase in lysozyme activity of yellow catfish (Pelteobagrus fulvidraco). Therefore, these results indicate that taurine not only can improve health and immunity of mammals (Kingston et al. 2004; Gupta et al. 2006), but also can improve the health and innate immunity of fish. IGF-1 mRNA expression was assessed in this study as a new parameter to determine whether taurine supplementation might have effect on gene expression. Interestingly, our results showed that IGF-1 mRNA expression level was significantly increased by 1.5% taurine supplementation. Some studies have been conducted to determine whether taurine supplementation could affect fish at gene expression level (Kingston et al. 2004; Gupta et al. 2006). The function of IGF-1 in fish is similar to that in human or mammals, and IGF-1 can only exert nutritional regulation in the liver of fish (Duan et al. 1994; Duan 1998). Therefore, only liver tissue was analyzed in this study for IGF-1 expression. Niu and Le Bail (1993) reported that IGF-1 level is significantly decreased in fasted rainbow trout. The elevated peak of IGF-1 in the present study suggests that IGF-1 gene can be up-regulated by the taurine supplementation in diets for olive flounder. Optimum level of taurine supplementation in fish diets has already been elucidated in previous studies for several fish species, such as cobia (Rachycentron canadum) (Kousoulaki et al. 2009), Japanese flounder (Kim et al. 2003, 2007, 2008), red seabream (Takagi et al. 2010), and sea bass (Brotons Martinez et al. 2004). According to these studies, the optimum level of taurine supplementation depends on fish species and growth stage of fish. Salze and Davis (2015) have reported that different taurine supplementation levels are needed in different fish species with different growth stages.
Conclusions
This study suggests that taurine supplementation is effective in improving growth performance, feed efficiencies and innate immunity of juvenile olive flounder in low water temperature season. The suggested taurine supplementation level would be between 1.0 and 1.5% (analyzed levels; 0.9–1.3% in diets) in a fish meal-based diet for olive flounder in suboptimal water temperature condition.