Background
The sea cucumber, Apostichopus japonicus, has become an important mariculture species in Russia, China, Japan, and South Korea because of its relatively high economic value (Sloan 1984). Market demand for this species increased because of its aphrodisiac and curative properties (Liao 1997). However, the production of sea cucumbers obtained from the natural environment has declined due to overexploitation and pollution (Conand 2004). Depletion of wild production together with high commercial value has encouraged the people to develop aquaculture methods for holothurians, especially A. japonicus (Conand 2004; Yuan et al. 2006).
Successful culture of juvenile sea cucumbers requires proper knowledge about feed intake behavior and dietary requirements (Slater et al. 2009). However, little is known regarding which artificial diets are capable of inducing rapid growth and healthy conditions of commercially important sea cucumbers (Slater et al. 2009; Yuan et al. 2006; Zhou et al. 2006).
Sea cucumbers are deposit feeders that ingest sediment containing organic matter, including bacteria, protozoa, diatoms, and detritus of plants or animals (Yingst 1976; Moriarty 1982; Zhang et al. 1995; Feng et al. 2016a, 2016b). A. japonicus preferentially inhabits the sea bottom in flourishing large algae, rich detritus of which provide sea cucumber with its main organic nutrient (Li et al. 1994; Zhang et al. 1995). Traditionally, sea cucumbers are cultured in earthen ponds without artificial feed. But recently, farmers have started to feed the sea cucumbers with formulated diets to increase production (Shi et al. 2013). Formulated diets for sea cucumbers are commonly made of macroalgal powder and sea mud. Among macroalgae, the brown algal Sargassum thunbergii is widely distributed over shallow coastal areas in Korea, Japan, and China and commonly used as a feed ingredient in sea cucumber culture (Sui 1989; Battaglene et al. 1999). However, it is difficult to satisfy demand for sea cucumber culture because this algal species is not produced commercially and its use as feed ingredients is also expensive (Lobban and Harrison 1994). In addition, more and more S. thunbergii have been harvested in recent years with the rapid expansion of sea cucumber farming scale, which results in severe damage to S. thunbergii resource (Yuan 2005; Wang et al. 2006).
Meanwhile, by feeding with commercial feed which mostly used S. thunbergii, sea cucumber have a high level of n-6 fatty acids and low n-3 fatty acids and the balance of the n-3/n-6 ratio is not good (Feng et al. 2016a, 2016b). But n-3 fatty acids and a good balance of the n-3/n-6 ratio is very important to protect from allergic and inflammatory diseases like asthma. So, reducing the S. thunbergii content of sea cucumber feed will be one strategy to increase the sustainability of the sea cucumber culture.
Therefore, it is critical to find good substitutes for S. thunbergii to relieve the pressure on natural S. thunbergii resource and produce good quality sea cucumber. Several researchers reported that juvenile sea cucumbers fed commercially available dried powdered macroalgae (Ulva lactuca, Laminaria japonica, Sargassum thunbergii, Sargassum polycystum) and sea mud exhibited significant growth (Battaglene et al. 1999; Liu et al. 2010; Zhu et al. 2007). In our study, we used Ulva lactuca, Undaria pinnatifida, Laminaria japonica, Nannochloropsis oculata, and Schizochytrium sp. as a partial alternative source of Sargassum thunbergii to produce significant growth and good quality sea cucumber. Ulva lactuca, Undaria pinnatifida, and Laminaria japonica are popular and cheaper algae in Korea and widely used in the culture of sea urchins and abalone (Agatsuma 2000, Qi et al. 2010). Nannochloropsis oculata and Schizochytrium sp. are considered promising algae for aquaculture and offer high levels of polyunsaturated fatty acids (PUFAs), especially eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) respectively (Kandilian et al. 2013 Yue et al. 2004).
Sea cucumbers have many therapeutic effects against various diseases (Bordbar et al. 2011; Guo et al. 2015). Moreover, sea cucumber extracts have potent biological effects and have antiviral, anticancer, antibacterial, antioxidant, and anti-inflammation effects (Esmat et al. 2013; Farshadpour et al. 2014; Kiani et al. 2014; Wijesinghe et al. 2013). In China and Malaysia, sea cucumbers have been traditionally used for the remedy of different inflammatory diseases like asthma. Asthma is a chronic inflammatory disease and a major public health problem. Interleukin (IL)-10 is a potent anti-inflammatory cytokine that downregulates the synthesis of Th1 (T helper 1)- and Th2 (T helper 2)-associated cytokines, chemokines, and inflammatory enzymes. It plays a vital role for the mitigation of allergic responses. But till now, there are no reports demonstrating the effect of different algae in sea cucumber on IL-10 production.
In this study, the effects of different algae in diet on growth, survival, and anti-inflammatory cytokine (IL-10) production of the juvenile sea cucumber were examined.
Methods
Six experimental diets designed as Sargassum thunbergii (ST), Ulva lactuca (UL), Undaria pinnatifida (UP), Laminaria japonica (LJ), Schizochytrium sp. (SS), and Nannochloropsis oculata (NO) were prepared. Ingredients and proximate compositions of experimental diets are presented in Table 1. Most sea cucumbers are deposit feeders that ingest sediment with organic matter. Several studies showed that juvenile sea cucumbers fed different algae and sea mud exhibited significant growth (Battaglene et al. 1999; Liu et al. 2009; Hai-Bo et al. 2015. ST diet was used as the control diet where 15% Sargassum thunbergii and 15% wheat flour were used. For diets UL, UP, LJ, SS, and NO, wheat flour was replaced by 15% UL, UP, LJ, SS, and NO respectively. All ingredients were ground into fine powder through a 200-μm mesh, thoroughly mixed, and stored at −20 °C.
aMineral premix (g kg−1 premix): MgSO4·7H2O, 80.0; NaH2PO4·2H2O, 200.0; KH2PO4, 130.0; Ferric citrate, 40.0; ZnSO4·7H2O, 10; Ca-lactate, 25.5; CuCl, 0.2; AlCl3·6H2O, 0.15; KIO3, 0.15; Na2Se2O3, 0.01; MnSO4·H2O, 2.0; CoCl2·6H2O, 1.0
bVitamin premix (g kg−1 premix): ascorbic acid, 92.7; α-tocopheryl acetate, 14.5; thiamine hydrochloride, 7; riboflavin, 7.0; pyridoxine hydrochloride, 1.4; niacin, 27.8; Ca-D-pantothenate, 9.7; myo-inositol, 139.1; D-biotin, 0.5; folic acid, 0.5; p-amino benzoic acid, 13.9; menadione, 4.2; retinyl acetate, 0.65; cholecalciferol, 0.8; cyanocobalamin, 0.004
The experiment was carried out for 9 weeks in the laboratory of Marine Biology and Aquaculture, Gyeongsang National University, Republic of Korea. Sea cucumbers used in this experiment were collected from the Goseong Sea cucumber farm. Prior to the experiment, sea cucumbers were transferred to the laboratory in fiberglass aquaria and acclimated for 2 weeks at 18 °C.
After 2 days starvation, 240 sea cucumbers with initial wet body weights of 2.98 ± 0.06 g (mean ± SE) were randomly selected from acclimatized sea cucumbers and placed in equal number into 24 fiberglass aquaria (45 × 60 × 50 cm3) to form six groups in tetraplicate. The six groups were fed with different experimental diets such as ST, UL, UP, LJ, SS, and NO respectively. A complete randomized block design was used to arrange the 24 aquaria of six treatment groups.
During the experiment, aeration was provided continuously and to ensure water quality two thirds volume of the water in each aquarium was exchanged every day. Seawater temperature was controlled at 18 ± 1.0 °C. Dissolved oxygen was maintained above 5.0–7.0 mg l−1, and the levels of ammonia in the water of the aquaria were less than 0.25 mg l−1. Other conditions were salinity 32 ± 1 ppt, pH 7.7–8.3, and photoperiod 24-h dark.
Twenty-four sea cucumbers were collected as an initial sample before starting the experiment. During the experiment, sea cucumbers were fed once per day (at about 1700 hours). Uneaten feed were collected by siphon after 24 h and then dried at 65 °C to constant weight. Sea cucumber feces were also collected by siphon once per day (1600 hours). The feces were dried at 65 °C to constant weight, and those from each aquarium were pooled for further analysis. At the end of the 9-week experiment, all the experimental sea cucumbers were deprived of food to clear their guts for 2 days, weighed, and then dried at 65 °C until constant weight was achieved.
Survival rate (SR), specific growth rate (SGR), ingestion rate (IR), feces production rate (FPR), and food conversion efficiency (FCE) were calculated as follows:
where N1 is the number of individuals alive at start of the experiment and N2 is the number of individuals alive at end of the experiment; W1 and W2 are initial and final combined dry weights, respectively, of all 10 sea cucumbers in each aquarium; T is the experimental period; I is the dry weight of the total feed ingested; and F is the dry weight of feces.At first, our experimental sea cucumbers were cleaned and the visceral organs removed. After that, the sea cucumbers were cut into small pieces and homogenized. One hundred fifty grams of samples were boiled in 300 ml distilled water for 20 min. After removing solid materials from the water, the boiled water was vaporized using a microwave until the mixture was reduced by 50%. After centrifugation of the extracts at 500×g for 10 min, a fivefold volume of 100% ethyl alcohol was added to the supernatant and incubated at 20 °C for 24 h. After that, the supernatant was discarded. The extract pellet was washed with 70% ethyl alcohol and centrifuged under the same conditions. The supernatant was discarded, and the pellet was evaporated under a vacuum. The final extracts were prepared by re-suspending the pellet in 20 ml distilled water (Lee et al. 2016).
In order to analyze the IL-10 gene expression, mice splenocytes were stimulated with 10 μg ml−1 of each experimental diet-fed sea cucumber extract for 2 h. The total RNAs were isolated by Qiazol reagent (Qiagen Science, USA) according to the manufacturer’s protocols. Two micrograms of total RNAs were transcribed using M-MLV reverse transcriptase (Promega, USA), according to the manufacturer’s protocols. IL-10 mRNA expression levels were synthesized by real-time PCR using the iCyclerTM (Bio-Rad Laboratories, Hercules, CA, USA). GAPDH was used for the reference gene. IL-10 and GAPDH primer sequence are previously described (Lee et al. 2016).
Statistical analysis was performed by the software SPSS 18.0 with possible differences among diet treatments being tested by using one-way ANOVA. Tukey multiple comparison tests were used to analyze the differences among treatments. Differences were considered significant at a probability level of 0.05.
Results
The growth performance and survival of sea cucumber are shown in Table 2. All sea cucumbers were alive at the end of the 9-week feeding trial. The growth performance of the sea cucumbers differed significantly among treatments. Final wet and dry body weights of sea cucumbers showed the highest value for the UL diet group and the lowest value for the ST diet group (P < 0.05).
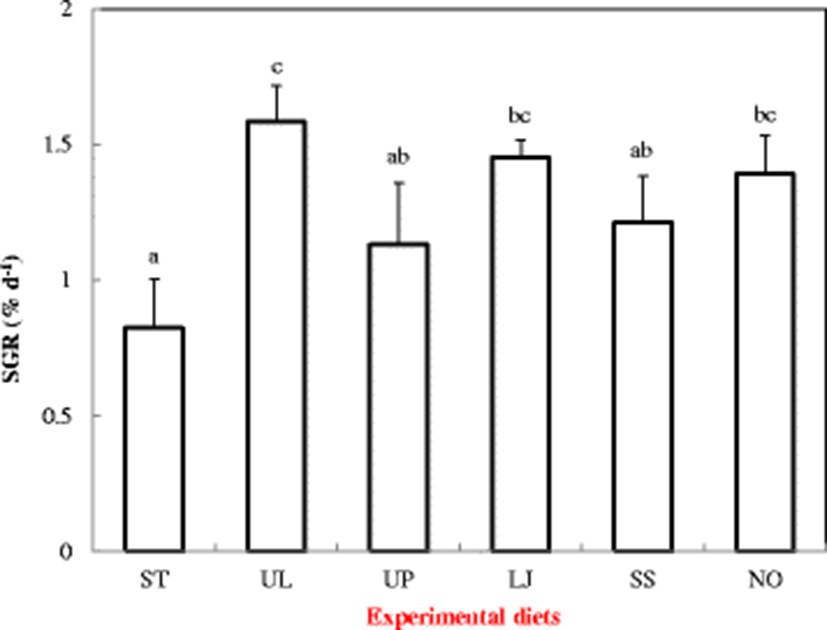
The highest SGR (1.58% d−1) was observed in sea cucumber fed the UL diet. SGR of sea cucumbers fed the ST diet was significantly (P < 0.05) lower than UL, LJ, and NO diets, but not significantly (P > 0.05) different from that fed the UP and SS diets (Fig. 1).
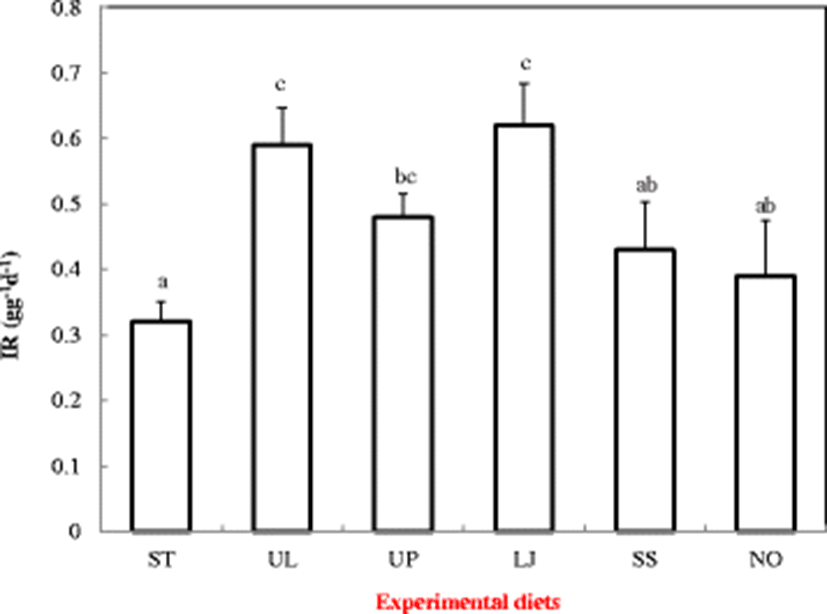
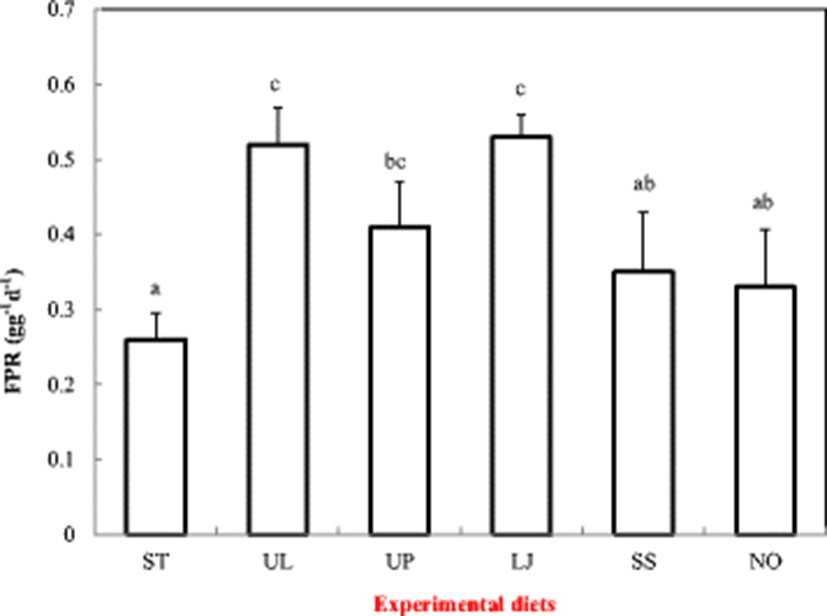
Ingestion rates (Fig. 2) and feces production rates (Fig. 3) of the sea cucumbers showed significant differences among different diet treatments. Sea cucumbers fed with diets LJ and UL showed significantly higher IR (0.62 and 0.59 g g−1 d−1 respectively) and FPR (0.53 and 0.52 g g−1 d−1 respectively) than those fed other diets (P < 0.05). Sea cucumbers fed with diet ST showed the lowest IR (0.32 g g−1 d−1) (P < 0.05) and FPR (0.26 g g−1 d−1) (P < 0.05) among all treatments.
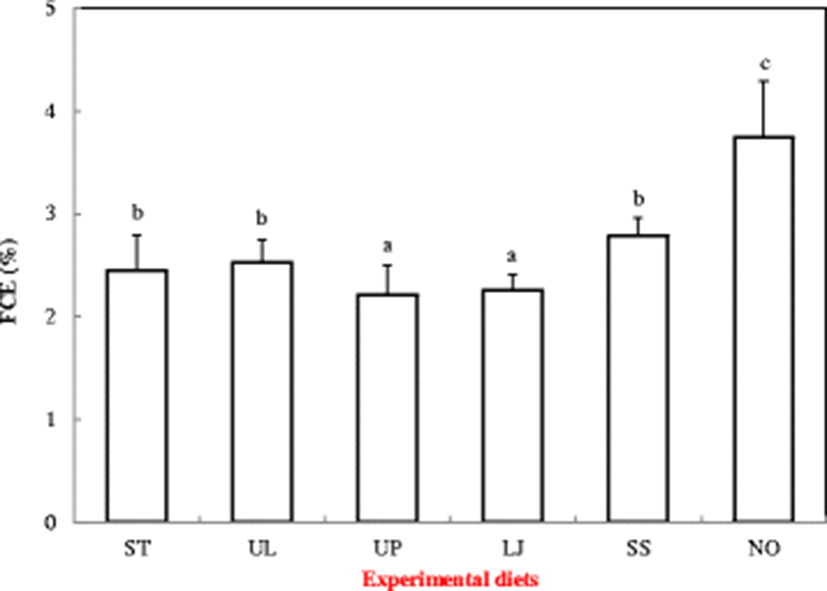
Food conversion efficiency (%) was significantly different among different diet treatments (Fig. 4). FCE of the sea cucumbers fed with diet NO was 3.74%, which was significantly higher than those fed with other diets (P < 0.05). Sea cucumbers fed the diet UP showed the lowest FCE (2.21%).
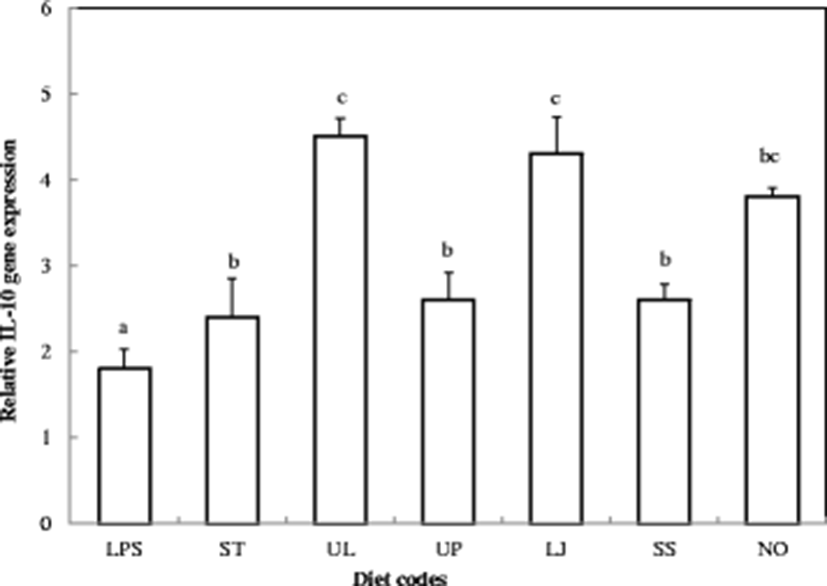
In order to establish proper algae for sea cucumber diet, we synthesize IL-10 gene expression levels. Splenocytes were stimulated with each experimental diet-fed sea cucumber extract for 2 h. Result showed that IL-10 gene expression levels were significantly increased in the UL, LJ, and NO diets compared to the other experimental diets (Fig. 5). The highest IL-10 gene expression levels were found when the sea cucumbers were fed the Ulva lactuca algae diet. However, IL-10 gene expression levels were not increased by ST, UP, and SS diets and have no significant differences. These results suggest that UL, LJ, and NO algae could upregulate IL-10 gene expression.
Discussion
In all treatments, no sea cucumber died and survival rates of sea cucumbers were excellent (100%) and were higher than the rates reported in previous similar studies (Hai-Bo et al. 2015; Slater and Carton 2007; Zhou et al. 2006). The results showed that sea cucumbers might have the ability to tolerate the different algae such as UL, UP, LJ, NO, and SS in diet.
Systematic studies on the use of algae as an ingredient in commercial feeds for sea cucumber culture are very rare. Many researchers have used different types of algae such as Sargassum polycystum, Sargassum thunbergii, Laminaria japonica, Spirulina platensis, Ulva lactuca, Undaria pinnatifida, and Pyropia spheroplasts to study sea cucumber nutrition requirements (Li et al. 2009; Liu et al. 2009; Seo and Lee 2011; Seo et al. 2011; Slater et al. 2009; Yuan et al. 2006, Shahabuddin et al. 2017). Most researchers have used S. thunbergii as a main feed ingredient in land-based intensive culture systems (Battaglene et al. 1999; Slater et al. 2009). However, in our study of various experimental diets, the SGR was much higher in sea cucumbers fed UL, LJ, or NO diets compared to UP, SS, and ST diets (Fig. 1). Seo et al. (2011) reported that sea cucumbers fed 20% L. japonica and 20% S. thunbergii containing diet grew much better than those only eating S. thunbergii (40%) diet. Zhu et al. (2007) also reported that the SGR was increased significantly when sea cucumbers were fed U. lactuca compared with S. thunbergii and the SGR of sea cucumbers decreased when they were fed L. japonica. They used much smaller sized (0.49 g) sea cucumbers compared to ours (2.98 g). Different size of sea cucumber may have different diet choice and nutrition requirement (Yanagisawa 1998).
Algae are an important food source for sea cucumbers. Zhu et al. (2007) reported that U. lactuca as a feed ingredient for the sea cucumbers was better than S. thunbergii in growth performance. In the present studies, NO, ST, SS, and UP diets of which the protein contents were higher than those of the UL and LJ diets were selected to test the effects on growth of the sea cucumbers (Table 2). The results showed that SGR of the sea cucumbers fed the UL and LJ diets was significantly higher than that of those fed the ST, SS, and UP diets. The results indicate that some other factors were responsible for the nutrient effects of the algae besides the protein, lipid, and energy contents. One of the three mechanisms such as acid hydrolysis, enzymatic digestion, or mechanical trituration is necessary to break the cell wall of microalgal cell contents (Bitterlich 1985). For instance, many fishes like tilapia are the species which rely on acid hydrolysis. They are able to disrupt cyanobacterium cell walls because of low pH values of their stomach fluid (Caulton 1976; Payne 1978). But for holothurians like the sea cucumber, the structure and environment of the digestive tract of the sea cucumber are quite different from tilapia. Sea cucumbers have no specialized organ for grinding or gland for chemical digestion (Massin 1982), and digestive enzyme activities are very low and have very little cellulose activity (Wang et al. 2007). Therefore, sea cucumbers are able to assimilate a specific amount of cellulose content.
In our studies, the higher SGR of the sea cucumber was observed in the treatments fed with the UL and LJ diets though the protein and lipid contents of those diets were comparatively low (P < 0.05). UL algae are two cells thick, soft and translucent, and LJ is multicellular, filamentous. Their cell walls are easy to break and have comparatively lower cellulose content (Burrows 1991; Miyai et al. 2008). So, sea cucumbers could easily digest and take full advantage of the nutrients in the UL and LJ algae.
The algae UP and SS contain a significant amount of cellulose and need specific enzyme activities to break the cellulose content (Jurkovic et al. 1995). Sea cucumbers have lower specific enzyme activities and may not digest macroalgae such as SS and UP efficiently (Wang et al. 2007). Moreover, Schizochytrium algae have a higher amount of lipids (Menghe et al. 2009). But sea cucumbers have low tolerance to lipids and do not require a high dietary lipid (Seo and Lee 2011). Therefore, in our studies, sea cucumbers fed the UP and SS diets had lower SGR among all the treatments with the exception of the ST diets.
Ingestion rates (IRs) of sea cucumbers were significantly affected by different experimental diets. There was a negative relationship between IR and the protein level. In the natural ecosystem, low nutritional value of sediment consumed by deposit feeders means those animals need to consume large amounts of sediment in order to obtain a net input of energy (Santos et al. 1994; Hudson et al. 2004). Vice versa, when food quality becomes better, internal appetite regulation would work actively to decrease food ingestion. In this study, the ingestion rate of sea cucumbers decreased when the protein content of the diets increased. The same phenomenon was also found in other echinoderms. McBride et al. (1998) reported that for sea urchin (Strongylocentrotus franciscanus), prepared diets of different protein levels resulted in different ingestion rate. Otero-Villanueva et al. (2004) also found in Psammechinus miliaris that the lowest ingestion rate was related to a high energetic diet.
Regulatory T cells (Treg cells), known as suppressor T cells, are a subpopulation of T cells and modulate the immune systems (Kikodze et al. 2016). IL-10 is one of the Treg cells and known as a key regulator of immunity to many infections or inflammatory diseases (Gutierrez-Murgas et al. 2016). For instance, high levels of IL-10 have protective effect against asthma disease (Raeiszadeh Jahromi et al. 2014). Conversely, lack of IL-10 promotes cell apoptosis during virus infection in the small intestine (Pan et al. 2014). In a previous study, we already investigated that administration of sea cumber total extract can upregulate IL-10 and ameliorate asthma disease (Lee et al. 2016). Here, we suggest that UL, LJ, and NO algae increase interleukin (IL)-10 gene expression.
Conclusions
In conclusion, the results of this experiment suggest that dietary inclusion with Ulva lactuca, Laminaria japonica, and Nannochloropsis oculata algae may improve growth of juvenile sea cucumbers and could upregulate IL-10 gene expression. Such detailed information could be helpful in further development of more appropriate diets for the culture of sea cucumber.