Background
Inflammatory response is a protective mechanism employed by living tissues against physiological and pathological antigens such as injurious and infectious stimulus (Oh et al. 2017). However, this process persists for a long period of time; it can lead to cardiovascular diseases, diabetes, arthritis, cancer, neurological diseases, pulmonary diseases, and autoimmune diseases (del Carmen Millán-Linares et al., 2014). Macrophages play a pivotal role as the host defense in the immune system (Jovanovic et al. 1998). In response to lipopolysaccharide (LPS) or interferon gamma (IFN-γ), macrophages produce and secrete nitric oxide (NO) and prostaglandin E2 (PGE2), which are synthesized by inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively, as well as pro-inflammatory cytokines such as including tumor necrosis factor-α (TNF- α), interleukin (IL)-1β, and IL-6 (Yoon et al. 2011).
Food protein hydrolysates generated by proteolytic enzyme treatment have been reported to possess nutraceutical potentials with beneficial human health. As the numerous studies have shown, the food protein hydrolysates have several biological activities, including anti-cancer (Nguyen et al. 2012), anti-hypertension (Ngo et al. 2015), osteoblastic differentiation (Nguyen et al. 2014), anti-inflammation, and anti-oxidant (Cattaneo et al. 2014). Among them, the health benefits of fish and shellfish have been reported with anti-inflammatory and anti-oxidative activity through production by enzymatic hydrolysis (Halldorsdottir et al. 2014; Holen et al. 2016; Qian et al. 2012).
Johnius belengerii is a commercial product with a high consumption of sea foods like fish cutlet in the USA, Australia, Europe, Korea, and Japan (Jung and Kim 2007). Although J. belengerii markets have quickly expanded, fishery byproducts including fish frame have rarely been used. According to the previous study, Kim et al. (2007) reported anti-oxidative effect of pepsinolytic hydrolysate from J. belengerii frame. However, it has not yet reported on anti-inflammatory activity of J. belengerii hydrolysate on LPS-stimulated macrophages. It is a superior candidate because oxidative stress was correlated with a wide spectrum of diseases, including chronic inflammation and cancers (Reuter et al. 2010). Therefore, the present study was investigated to evaluate the anti-inflammatory effects of J. belengerii frame hydrolysate (JFH) in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages, as well as the mechanism underlying this effect.
Methods
J. belengerii frames were donated from Charmson Food Co. Ltd. (Busan, Korea). Pepsin from porcine gastric mucosa, 3-(4, 5–dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) Griess reagent, and LPS were purchased from Sigma Aldrich (Sigma Aldrich, St. Louis, USA). Dulbecco’s minimum Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin, and other materials used in cell culture experiment were purchased from GIBCO™, Invitrogen Corporation, USA. The specific antibodies used for Western blot analysis were purchased from Santa Cruz Biotechnology (Santa Cruz, Ca, USA) and Ameresham pharmacia biosciences (Piscataway, NJ, USA).
The fish frame powder was digested with pepsin (enzyme/substrate ratio, 1/100; substrate concentration, 1%) in 5% acetic acid solution (pH 2.0) according to our previous method (Jung and Kim 2007). After incubation at 100 °C for 10 min to inactivate enzyme, the solution was filtered through an ashless Whatman No. 41 filter paper. After removing insoluble materials by centrifuging at 3000×g for 20 min, soluble hydrolysate in supernatant was lyophilized to obtain a JFH dry powder. The JFH was kept − 70 °C for further experiments.
RAW 264.7 macrophages were obtained from the American Type of Culture Collection (Rockville, MD, USA). Cells were grown in DMEM supplemented with 10% FBS, 1% penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37 °C in 5% CO2-humidified air environment. Cells were sub-cultured every 3 days.
MTT assay was performed to assess the cytotoxicity of RAW 264.7 macrophages. Briefly, cells were seeded on the plate and culture with various concentrations of the JFH (10, 50, and 100 μg/ml) for 24 h. MTT solution was added at each well. After incubation for 4 h, the supernatants were aspirated. The formazan crystal was dissolved in dimethyl sulfoxide (DMSO), and the absorbance was measured with a microplate reader (PowerWave XS2, BioTek Instruments, Inc., USA) at 550 nm.
NO levels in the culture supernatants were determined by measuring nitrite, which is a major stable product of NO, using the Griess reagent. After the pre-incubation of the RAW 264.7 macrophages with various concentrations of the JFH and LPS (0.25 μg/ml) for 24 h, the quantity of nitrite accumulated in the culture medium was measured as an indicator of NO production. Briefly, 100 μl of cell culture medium was mixed with 100 μl of Griess reagent; the mixture was incubated at room temperature for 10 min, and the absorbance at 540 nm was measured in a microplate reader. Fresh culture medium was used as a blank in every experiment.
The cells were seeded in 6-well culture plates at a density of 1 × 106 cells/well and grown in 2 ml of DMEM for 24 h. To determine iNOS and COX-2, cells were stimulated with LPS (0.25 μg/ml) in the presence of JFH (10, 50, and 100 μg/ml) for 24 h. On the other hand, signal pathways (MAPKs and NF-κB) determined that cells were stimulated with LPS (0.25 μg/ml) in the presence of JFH (10, 50, and 100 μg/ml) for 30 min. After that, all steps are same to following below processes. Then, the cells were collected and washed twice with PBS. The cells were lysed in lysis buffer (20 mM Tris, 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 1% NP-40, 10 mg/ml aprotinin, 10 mg/ml leupeptin, and 1 mM PMSF) for 60 min and then centrifuged at 16,000×g and 4 °C for 15 min. The protein concentrations were determined using the BCA™ protein assay kit. The lysate containing 40 μg of protein was subjected to electrophoresis on a sodium dodecyl sulfate (SDS)-polyacrylamide gel, and the gel was transferred onto a nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in TBS-T (25 mM Tris-HCl, 137 mM NaCl, 2.65 mM KCl, and 0.05% Tween 20, pH 7.4) for 2 h. The primary antibodies (glyceraldehyde 3-phosphate dehydrogenase; GAPDH, Lamin B, iNOS, COX-2, p-ERK, ERK, p-p38, p38, p-JNK, JNK, p-I-κB, I-κB, p65, and p50) were used at a 1:1000 dilution. The membranes were incubated with the primary antibodies at 4 °C overnight, washed with TBS-T, and then incubated with the secondary antibodies at 1:3000 dilutions. The signals were developed using an ECL Western blotting detection kit and quantified using Davinci K chemi-doc imaging system (Young Hwa scientific Co. LTD., Seoul, Korea), and protein expression was quantified by Image J software (Wayne Rasband, NIH, Bethesda, Md., USA).
The cells were seeded in 6-well culture plates at a density of 1 × 106 cells/well and grown in 2 ml of DMEM for 24 h. The total RNA from the LPS-stimulated RAW 264.7 macrophages in the presence or absence of the JFH was extracted using the TRIzol reagent. After treatment of TRIzol, the suspension was mixed with chloroform and centrifuged at 18,000×g for 10 min at 4 °C. The aqueous phase was separated and RNA in the phase was precipitated by mixing with isopropyl alcohol and centrifuged at 18,000×g for 10 min at 4 °C. The precipitate was washed with 75% ethanol, dried, and then dissolved in diethyl pyrocarbonate (DEPC) containing water. The total RNA content was calculated based on the absorbance at 260 nm, and the quality was confirmed. Equal amounts of RNA were used for each cDNA synthesis reaction according to the manufacturer’s instruction. Then, 2 μl cDNA was used for reverse transcription and amplified by PCR using the access RT-PCR system. PCR was performed using primers for mouse iNOS, COX-2, TNF-α, IL-1β, IL-6, and GAPDH. The PCR was performed using selective primers for the iNOS (5′-ATGTC CGAAGCAAACATCAC-3′ and 5′-TAATGTCCAGGAA GTAGGTG-3′), COX-2 (5′-GGGGTACCTTCCAGCTGTCAAAA TCTC-3′ and 5′-GAAGATCTCGCCAGGTACTCACCTG-3′), TNF-α (5′-ATGAGCACAGAAAGCATGA TC-3′ and 5′-TACAGGCTTGTCACTCGAATT-3′), IL-1β (5′-ATGGCAACTGTTCCTGAACTCAACT-3′ and 5′-TTTCCTTTCTTAGATATGGACAGGAC-3′), IL-6 (5′-ATGAGCACAGAAAGCATGATC-3′ and 5′-TACAGGCTTGTCACTCGAATT-3′), and GAPDH (5′-TTTGTGATGGGTGTGAACCACGAG-3′ and 5′-GGAGACAACCTGGTCCTCAGTGTA-3′). The following PCR conditions were applied for all amplifications by 25 cycles of 95 °C for 30 s (denaturing), 58 °C for 30 s (annealing), and 72 °C for 30 s (primer extension). Following amplification, portions of the PCR reactions were electrophoresed on an agarose gel for 20 min at 100 V. The gels were visualized after staining with EtBr using UNOK-8000s Gel Manager System (Biotechnology, Seoul, Korea), and mRNA expression was quantified by Image J software.
Results and discussion
In accordance with our previous study, the JFH was mainly composed of 63.95% protein (Jung and Kim 2007). Compared with previous studies, protein content of JFH was determined lower than Sardina pilchardus (85.8%), Trachurus mediterraneus (86.3%), Mallotus villosus (72.4%), Clupea harengus (77.0%), and Salmo salar (88.39%) because fish frame contains amount of ash (Chalamaiah et al. 2012; Morales-Medina et al. 2016). To prepare a soluble hydrolysate without inorganic precipitate, we performed centrifugation to remove insoluble materials and filtration through 0.2-μm syringe filter (Toyo Roshi Kaisha Ltd., Tokyo, Japan). Then, protein concentration of the soluble hydrolysate was measured. At the result, we determined that the soluble hydrolysate consists of 97.9 ± 0.87% protein concentration and used further experiments.
Prior to evaluation of the NO inhibitory effect of JFH, we first examined its cytotoxic effect in LPS-stimulated RAW 264.7 macrophages using MTT assay. As shown in Fig. 1a, no significant cytotoxic effects were observed in RAW 264.7 macrophages, which were treated with various concentrations of JFH (10, 50, and 100 μg/ml) in the presence of LPS. Thus, those concentrations were used in subsequent experiments.
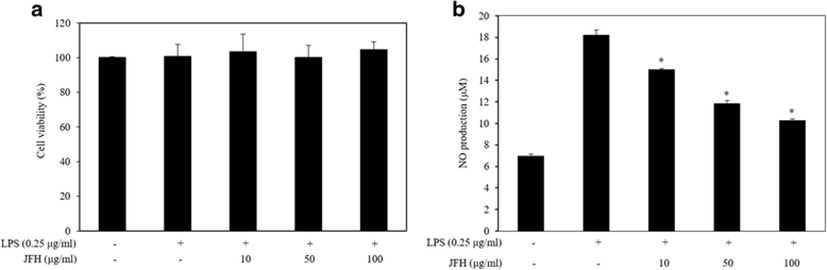
To evaluate whether the JFH suppressed the production of LPS-stimulated NO, RAW 264.7 macrophages were stimulated with LPS in the absence or presence of the JFH. The result shows that LPS treatment alone markedly induced NO production by the treated cells compared with untreated cells. However, treatment of the various concentrations of JFH (10, 50, and 100 μg/ml) significantly reduced NO production in a concentration-dependent manner (Fig. 1b). The investigation of NO inhibitory effect of protein hydrolysates has already been reported from marine organisms such as Mytilus coruscus (Kim et al. 2013), Ruditapes philippinarum (Lee et al. 2012), Crassostrea gigas (Hwang et al. 2012), Haliotis discus hannai (Qian et al. 2012), and Styela clava (Ko and Jeon 2015). Compared to what has been observed in previous studies, JFH exhibited a similar NO inhibitory effect compared to the hydrolysate of Haliotis discus hannai at the same concentration (Qian et al. 2012) and a more effective NO inhibitory effect than the sweetfish and Mytilus coruscus hydrolysates at the same concentrations (Sung et al. 2012; Kim et al. 2013) in LPS-stimulated RAW 264.7 macrophages.
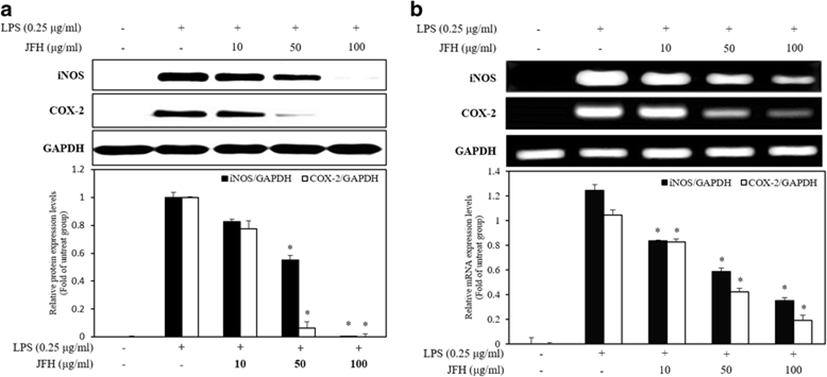
NO plays an important role in the immune system. It is produced from L-arginine by the three-member enzyme family including iNOS, endothelial NOS (eNOS), and neuronal NOS (nNOS) (Ryu et al. 2014). In the pathological conditions, potent inflammatory mediators, macrophages, were activated and secreted amounts of NO (Choi et al. 2014). According to previous studies, iNOS inhibitors have a similar structure of arginine or exhibit competitive inhibition pattern (Bonnefous et al. 2009). Therefore, we suggest that JFH contains the component of the similar structure to arginine and it has the potential to inhibit iNOS. Moreover, COX-2 also plays a pivotal role that it controls the immune homeostasis including inflammatory response and cancer immune evasion (Wehbi and Taskén 2016). Although NO plays a critical role in defending the pathogen, it can lead to an excessive inflammation and tissue damage due to NO overproduction (Segovia et al. 2013). Therefore, modulation of the inflammatory mediators such as iNOS and COX-2 has been shown to reduce the inflammatory diseases. To determine the mechanism involved in the suppression of inflammatory mediators by the JFH on LPS-stimulated RAW264.7 macrophages, we investigated the effect of the JFH on iNOS and COX-2 protein (Fig. 2a) and mRNA (Fig. 2b) expression by Western blotting and RT-PCR analysis. In response to LPS, the protein and mRNA expression of iNOS and COX-2 was markedly increased compared with the untreated cells. However, JFH significantly reduced the levels of the iNOS and COX-2 protein and mRNA expression in a concentration-dependent manner. JFH has more effectively inhibited the levels of iNOS and COX-2 expressions compared to Ruditapes philippinarum (Lee et al. 2012) and Styela clava flesh tissue (Ko and Jeon 2015) hydrolysates. From the results of the above studies, JFH is believed to have a more effective anti-inflammatory effect due to NO inhibition than other marine organisms.
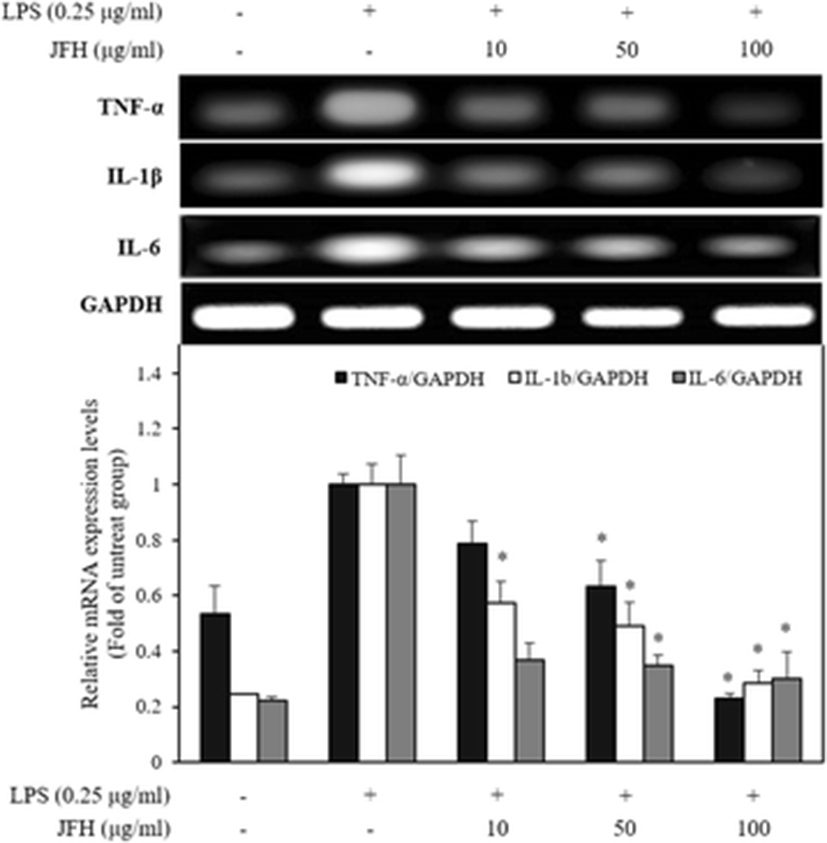
The pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, affect immune cell function, proliferation, and activation (Ahn et al. 2015). According to the Dragan et al. (1998), pro-inflammatory cytokines were closely related to the inflammatory response that they induced NO by activation of the iNOS. Moreover, Nrf2 is the key regulator of suppressing cytokines by LPS-induced oxidative stress like NO on the macrophages (Kobayashi et al. 2016). Among them, TNF-α is mainly released from LPS-induced macrophages and induces apoptosis, chronic inflammation, and associated diseases (Kim et al. 2011). Moreover, it induces the physiological effects such as septic shock and cytotoxicity as well as inducing IL-1β and IL-6 (Ryu et al. 2014). IL-1β possesses various biological properties and has been shown to induce the inflammatory and immunological responses by releasing prostaglandins (Ligumsky et al. 1990). Multifunctional cytokine of IL-6 also regulates the immune response, hematopoiesis, and inflammation (Ishihara and Hirano 2002). Ishihara and Hirano (2002) reported that IL-6 involves some diseases such as autoimmune diseases, chronic inflammatory proliferative disease, and B cell malignancy. To evaluate the inhibitory effect of the JFH on the pro-inflammatory cytokine production, we performed the expression of mRNA levels of the pro-inflammatory cytokines in the presence of the LPS. As shown in Fig. 3, LPS treatment alone dramatically increased TNF-α, IL-1β, and IL-6 mRNA expression levels by the treated cells compared with untreated cells. The JFH significantly suppressed TNF-α, IL-1β, and IL-6 mRNA expression levels by LPS-stimulated RAW 264.7 macrophages. These inhibitory effects on pro-inflammatory cytokine transcription and above-described NO inhibition demonstrated that JFH probably has anti-inflammatory efficacy by inhibiting Nrf2 as well as below described MAPK and NF-κB. Moreover, JFH has an inhibitory effect of TNF-α and IL-1β similar to salmon hydrolysate, but inhibitory effect of IL-6 is better (Ahn et al. 2012). Moreover, JFH has more effectively suppressed cytokine production compared to Plecoglossus altivelis (Sung et al. 2012).
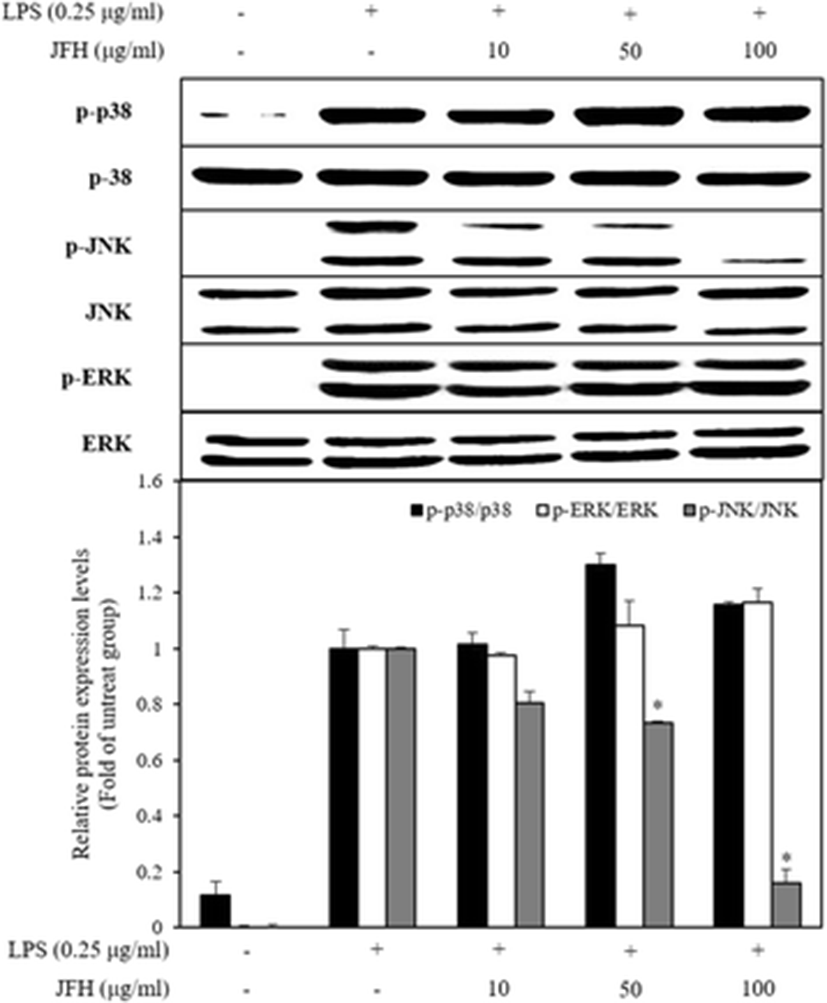
MAPKs, including p38 MAPKs, extracellular regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), play an important role that modulated a wide range of physiological processes such as cell growth, differentiation, and cellular response to cytokines through the intracellular signaling cascades (Jeong et al. 2016). The MAPK pathway sequentially activates MAP kinase kinase kinase (MAP3K), MAP kinase kinase (MAP2K), and MAPK (Dennler et al. 2000). During inflammatory processes, MAPK is involved in the synthesis and secretion of inflammatory mediators by LPS stimulation (Kim et al. 2016). According to the previous reports, p38 MAPK possesses the multifarious functions and regulates the expression of iNOS and COX-2 as well as the secretion of TNF-α in the LPS-induced macrophages (Jeong et al. 2016). ERK also is involved in the production of TNF-α on LPS-induced macrophages (Yuan et al. 2016). Furthermore, JNK is activated by the environmental stress and has implicated the iNOS expression in LPS-stimulated RAW 264.7 (Jeong et al. 2016; Ryu et al. 2014). Thus, MAPK inhibition is a key mechanism for suppression of inflammatory diseases. To elucidate the inhibitory mechanism of the inflammation response, we investigated the effects of the JFH on the activation of MAPKs in LPS-stimulated RAW 264.7 macrophages. The JFH significantly decreased the phosphorylation of LPS-stimulated JNK but not that of p38 and ERK (Fig. 4). JNK phosphorylates several transcription factors including c-Jun, activating transcription factor 2 (ATF-2), and Elk-1 for translocation into the nucleus. Particularly, c-Jun binds in the DNA binding site in the form of the homodimer or heterodimer with Fos family and control expression of the pro-inflammatory factors (Dennler et al. 2000). Therefore, we suggest that JFH inhibits transcription factor through phosphorylation of JNK and probably affects MAP3K or MAP2K.
NF-κB pathway is also a major signaling pathway implicated in regulating the inflammatory mediators in macrophages (Yeom et al. 2015). In LPS-stimulated macrophages, inhibitory factor kappa B (I-κB) is phosphorylated, ubiquitinated, and degraded by the proteasome (Jeong et al. 2016). Thereby, releasing NF-κB from I-κB-NF-κB complex translocates to the nucleus. The translocated NF-κB is involved in the production of pro-inflammatory cytokine, IL-1β, and TNF-α and suppression of anti-inflammatory mediators (Tak and Firestein 2001). Furthermore, NF-κB downregulated by phosphorylation of phosphatidylinositol-3-kinase (PI3K)/Akt and MAPKs as well as anti-oxidant (Kim et al. 2016). Particularly, PI3K/Akt signaling produces reactive oxygen species (ROS) that affects NF-κB activation. Therefore, inhibition of the translocation of NF-κB through suppressing I-κB phosphorylation and PI3K/AKT activation is implicated in the treatment of inflammatory diseases.
Figure 5 shows that LPS treatment alone markedly increased phosphorylation and degradation of I-κB in the cytosol and increased translocation of NF-κB (p65 and p50) in the nucleus, whereas LPS treatment with the JFH significantly decreased phosphorylation of I-κB in the cytosol and decreased translocation of NF-κB (p65 and p50) in the nucleus. Based on the results, JFH affects NF-κB translocation via JNK phosphorylation and anti-oxidative activity (Kim et al. 2007) as above described. In addition, it probably suppresses activation of PI3K/Akt signal pathway. In comparison with the previous study, 100 μg/ml of JFH has similarly effectively inhibited translocation of NF-κB by degrading I-κB to 200 μg/ml of Plecoglossus altivelis (Sung et al. 2012). Therefore, JFH has an effective anti-inflammatory activity potential due to inflammatory-mediated factors through inhibition of the NF-κB translocation.
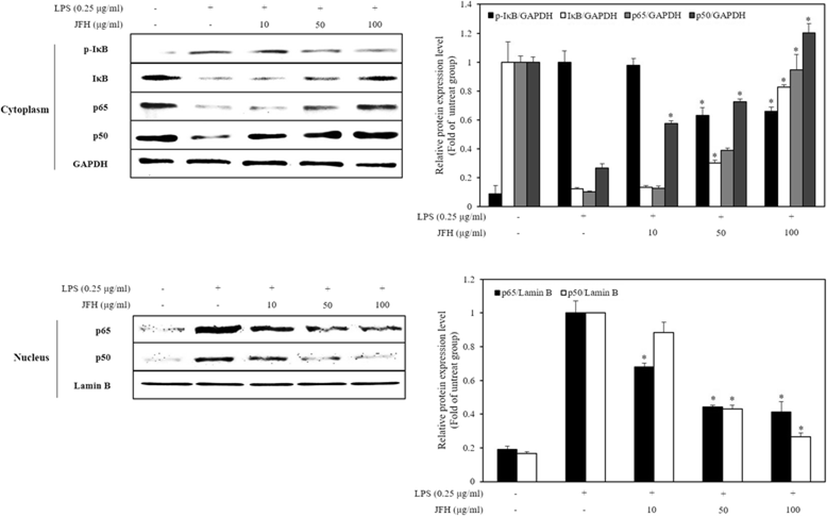
Conclusions
In the present study, we evaluated the anti-inflammatory effect of the J. belengerii frame hydrolysate (JFH) in LPS-stimulated RAW 264.7 macrophages. Based on the results, the JFH exhibited anti-inflammatory activities by reduction of cytokines, iNOS, and COX-2 protein and mRNA expression through inhibition of I-κB degradation and NF-κB translocation and phosphorylation of JNK not p38 and ERK. These results suggest that the JFH could be potential candidates to develop functional foods and pharmaceutical products for the treatment of the inflammatory diseases.