Background
The marine red algal genus Hypnea J.V. Lamouroux, which has a wide geographical distribution around the world, is economically important as food and as one of the sources of carrageenan, particularly in the tropics (Mshigeni and Chapman 1994; Geraldino et al. 2010). This genus was established based on six species (Lamouroux 1813) but later lectotypified by H. musciformis (Wulfen) J.V. Lamouroux (Kylin 1930; Papenfuss 1958).
Agardh (1852) proposed three sections within the genus Hypnea on the basis of thallus habit. The sect. Pulvinatae has creeping thalli of intricate cushion-like mat form. The sect. Virgatae is characterized by an erect, caespitose, and non-intricate thallus with main axis and dense lateral branchlets, while the sect. Spinuligerae in which the species show intricate and caespitose thalli with alternate branching and short spine-like branchlets. Recently, this infrageneric taxonomic scheme, with some exceptional species, was strongly supported by molecular analyses (Geraldino et al. 2010).
Since Lamouroux (1813), many species have been reported in this genus (Agardh 1851, 1852; Tanaka 1941, 1960; Schneider and Searles 1976; Nauer et al. 2014, 2015; Geraldino et al. 2009, 2010; Jesus et al. 2013). Therefore, Hypnea is a large genus in which 60 species are currently accepted within the family Cystocloniaceae (Guiry and Guiry 2018). This genus is characterized by a terete to compressed, branched thalli with short lateral branchlets, protuberant globular cystocarps, and zonately divided tetrasporangia on short lateral branchlet (Lamouroux 1813; Mshigeni 1978; Womersley 1994). However, species delimitation is complicated by the high degree of morphological variation within individual species, which may be chiefly influenced by environmental factors occurring in specific habitats. Furthermore, the taxonomy based mainly on vegetative features, with a relatively simple and plastic morphology, has caused many difficulties in identifying the species within Hypnea (Nauer et al. 2014, 2016; the present study).
In Korea, 13 species have been reported. During a survey of marine algal flora, three gigartinalean species were collected from Korea. They share the generic morphological features of Hypnea. The Hypnea species are newly recorded in Korea based on the morphological and molecular analyses herein.
Methods
Specimens for this study were collected from Pohang and Sacheonjin located on the eastern coasts of Korea. Taxonomic data were obtained from fresh, liquid-preserved, and herbarium specimens. Liquid-preserved material was stored in a 10% solution of formalin/seawater. Blades dissected from the cleared materials were hand sectioned, transferred to a slide with distilled water, and mounted in pure glycerin. Measurements are given as width and length. For permanent slides, the glycerin was exchanged with 10–20% corn syrup.
Total genomic DNA was extracted from silica gel-preserved samples using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Before extraction, dried material was crushed with liquid nitrogen using a mortar and pestle. Extracted DNA was used for amplification of ribulose-1, 5-bisphosphate carboxylase large subunit (rbcL) regions. For rbcL, the gene was amplified in three overlapping parts with the primer pairs FrbcL start (5′-TGTGTTGTCGACATGTCTAACTCTGTAGAAG-3′) - R753 (5′-GCTCTTTCATACATATCTTCC-3′), F492 (5′-CGTATGGATAAATTTGGTCG-3′) - R1150 (5′-GCATTTGTCCGCAGTGAATACC-3′), and F993 (5′-GGTACTGTTGTAGGTAAATTAGAAGG-3′) - RrbcS (5′-TGTGTTGCGGCCGCCCTTGTGTT AGTCTCAC-3′) (Freshwater and Rueness 1994). PCR amplifications were performed in a TaKaRa PCR Thermal Cycler Dice (TaKaRa Bio Inc., Otsu, Japan). PCR was performed with an initial denaturation step at 94 °C for 10 min, followed by 35 cycles of 30 s at 90 °C, 30 s at 50 °C, and 2 min at 72 °C, with a final 10-min extension cycle at 72 °C. The PCR products were moved to Macrogen Sequencing Service for sequencing (Macrogen, Seoul, Korea). The PCR primers were also used for sequencing.
Six rbcL sequences were generated. A total of 31 sequences from Hypnea, including Cystoclonium purpureum (Hudson) Batters as an outgroup, was aligned using 1356 base pairs (bp) of the rbcL gene using BioEdit (Hall 1999). Phylogenetic analyses were performed using neighbor-joining and maximum-likelihood methods using Mega 6 program. Bootstrap values were calculated with 1000 replications. RbcL sequences of other species were obtained from GenBank.
Results
Type locality: Uji Islands, Kagoshima Prefecture, Japan (Yamagishi and Masuda 1997)
Korean name: Mit-eong-kin-ga-si-u-mu nom. nov. (신칭: 밑엉킨가시우무)
Specimens examined: NIBRAL0000146496, MGARBb000744, MGARBb000745, MGARBb000746 (Daebori: 07.viii.2014)
Habitat: Epilithic in upper to lower intertidal
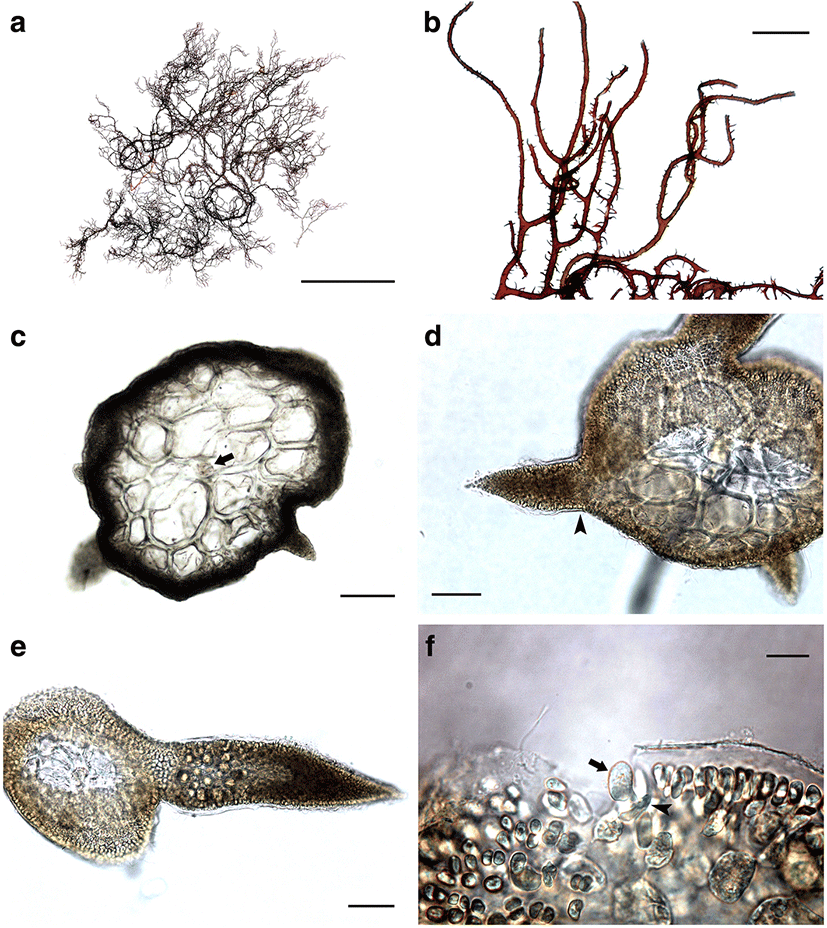
Morphology: Thalli up to 10–15 cm high, epilithic, subcompressed or subterete to terete, somewhat entangled at base, without iridescence, light brown to dark brown in color, cartilaginous in texture (Fig. 1a); main axes more or less percurrent, issuing irregularly or alternately branches with wide angle and proliferations; branches bearing a few branchlets in alternate to spiral manner (Fig. 1b); branchlets short, spinous, slender, rarely hooked, without constriction near base, 5–20 mm long (Fig. 1d); apical cells distinct at the apices of axes (Fig. 1c); lenticular thickenings usually absent in the wall of medullary cells; cortex 2–3 cell layers thick; medullary cells round to elliptical in transverse section, linear to cylindrical shape in longitudinal section, with many pit connection between adjacent cells; tetrasporangia produced from cortical cell (Fig. 1f), restricted in ultimate branchlets (Fig. 1e), zonately divided, 25–35 × 55–70 μm. Sexual plants were not collected during the present study.
Type locality: ad oras Novae Hollandiae (Silva et al. 1996)
Korean name: God-eun-ga-si-u-mu nom. nov. (신칭: 곧은가시우무)
Specimens examined: NIBRAL0000146479, MGARBb000747, MGARBb000748, MGARBb000749 (Sacheonjin: 03.vii.2014)
Habitat: Epilithic in upper to lower intertidal
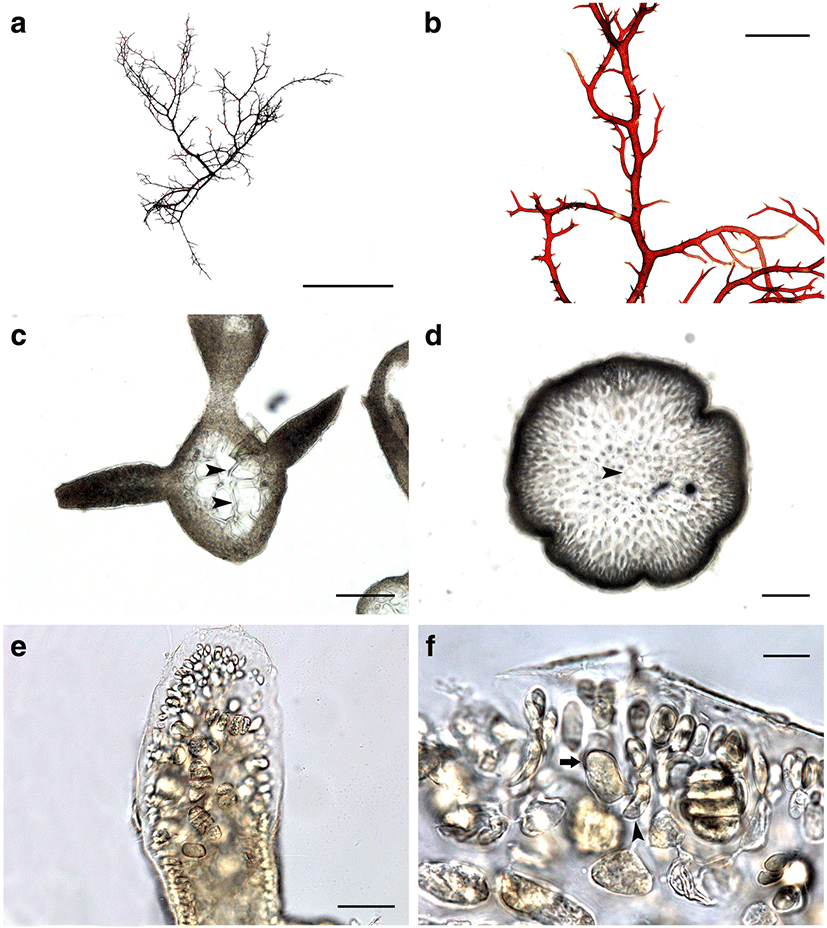
Morphology: Thalli up to 10–15 cm high, epilithic, terete, somewhat entangled at basal part, without iridescence, dark red to brown in color, cartilaginous in texture (Fig. 2a); main axes often more or less percurrent, issuing irregular or alternate branches and proliferations; branches bearing abundant branchlets in alternate to spiral manner (Fig. 2b); branchlets short, spinous, usually with constriction near base, 5–15 mm long; apical cells distinct at the apices of axes (Fig. 2d); lenticular thickenings occasionally present in the medullary cell walls (Fig. 2c); cortex 2–3 cell layers thick; medullary cells round to elliptical in transverse section, linear to cylindrical shape in longitudinal section, with many pit connection between adjacent cells; tetrasporangia produced from cortical cell (Fig. 2f), restricted in ultimate branchlets (Fig. 2e), zonately divided, 10–20 × 20–40 μm. Sexual plants were not collected during the present study.
Hypnea nidulans Setchell 1924: 161
Type locality: Tutuila Island, American Samoa (Silva et al. 1996; Setchell 1924)
Korean name: Gi-neun-ga-si-u-mu nom. nov. (신칭: 기는가시우무)
Specimens examined: NIBRAL0000146497, MGARBb000741, MGARBb000742, MGARBb000743 (Sachenjin: 20.vii.2017)
Habitat: Epilithic or occasionally epiphytic in upper to lower intertidal
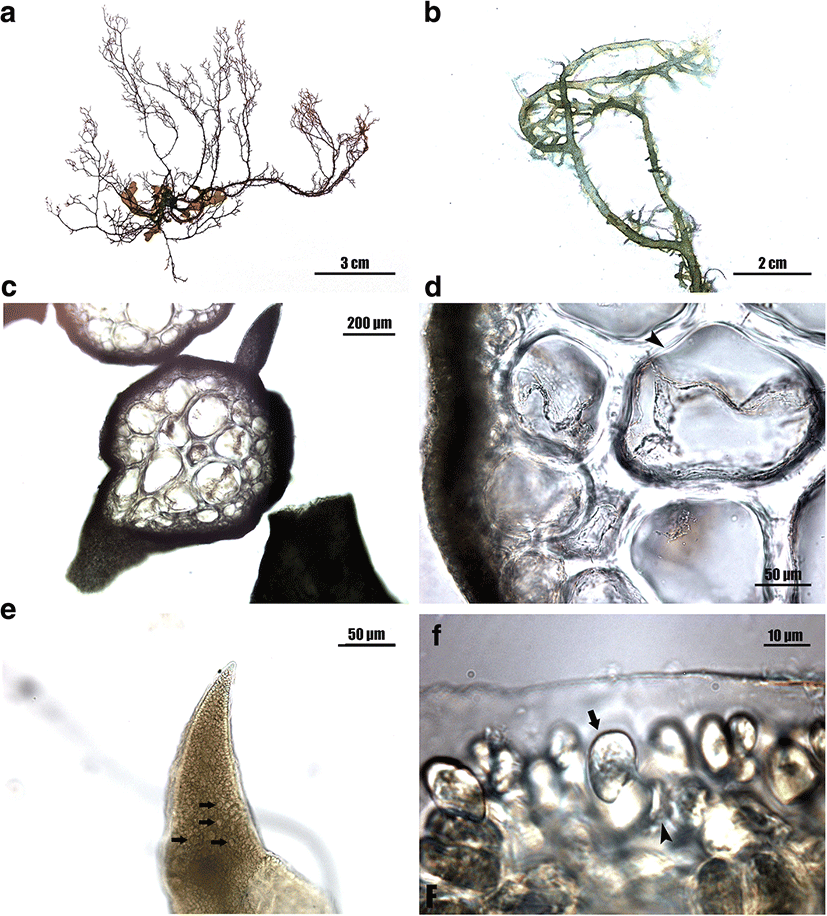
Morphology: Thalli up to 5–10 cm high, interwoven, loosely entangled, terete to subterete, without iridescence, red to brown in color, cartilaginous in texture (Fig. 3a); main axes erect, often percurrent, issuing irregularly branches and proliferations; branches bearing numerous branchlets in alternate to spiral manner (Fig. 3b); branchlets short, linear to lanceolate, usually with constriction near base, 2–10 mm long; apical cells distinct at the apices of axes; lenticular thickenings present in the wall of medullary cells (Fig. 3c, d); cortex 2–3 cell layers thick; medullary cells round to elliptical in transverse section, linear to cylindrical shape in longitudinal section, with many pit connection between adjacent cells; tetrasporangia produced from cortical cell (Fig. 3f), restricted in ultimate branchlets (Fig. 3e), zonately divided, 20–30 × 45–60 μm. Sexual plants were not collected during the present study.
Discussion
In a phylogenetic tree based on rbcL sequences, three major clades were confirmed (Fig. 4). These clades appear to be supported by the sectional features suggested by Agardh (1852).
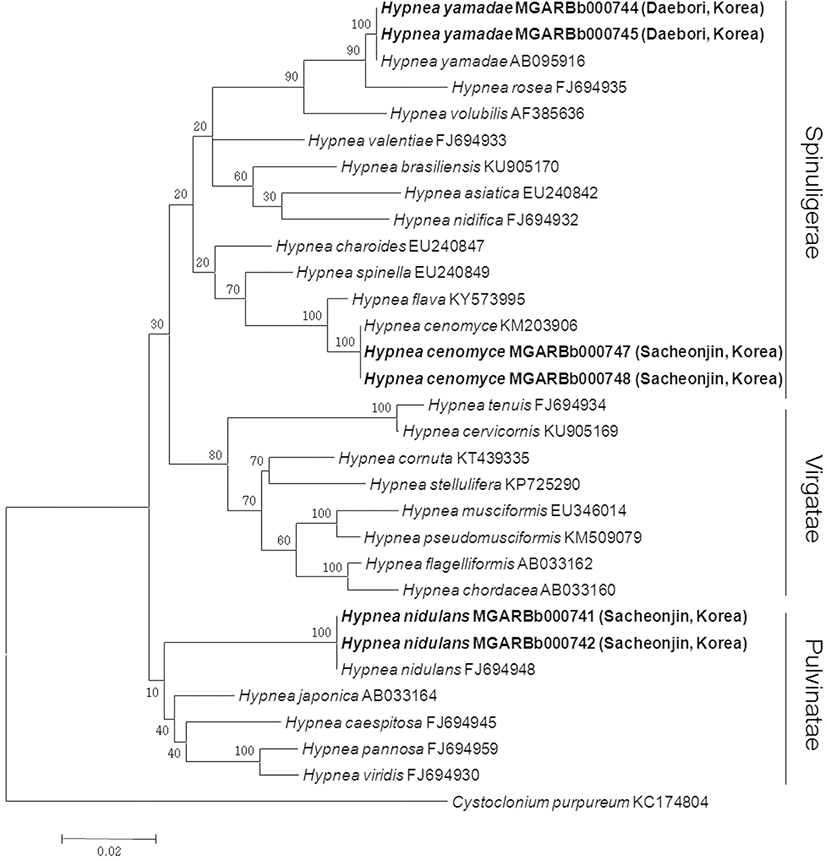
The first species, which belongs to a species group corresponding to the sect. Spinuligerae, nests in the same clade with H. yamadae in a genetic distance of 0% (Fig. 4). It is also morphologically characterized by an entangled base of creeping branches, subcompressed or subterete to terete axes, somewhat percurrent main axis, irregular or alternate branching with wide angle, and rarely hooked spinous branchlets as H. yamadae reported previously by some authors (Tanaka 1960; Yamagishi and Masuda 1997). However, somewhat terete thalli and slender branchlets rarely hooked in the species differed from the original description of H. yamadae (Tanaka 1960) (Table 1). It was distinguished from its sister species, H. rosea Papenfuss (1947), by terete branch rather than subcompressed or subterete to terete one.
|
Characters |
Species |
||
|---|---|---|---|
|
H. yamadae |
H. cenomyce |
H. nidulans |
|
|
Habitat |
Epilithic in intertidal to subtidal |
Epilithic in intertidal |
Epilithic or occasionally epiphytic in intertidal |
|
Thalli |
Terete to compressed |
Terete |
Terete to subterete |
|
Size (cm) |
9–37 |
4–15 |
2–10 |
|
Branching type |
Irregular, alternate or dichotomous |
Alternate or irregular |
Alternate to spiral, irregular |
|
Branchlets |
A few |
Several (or abundant) |
Numerous |
|
Basal constriction of branchlets |
Absent |
Present |
Present |
|
Hooked branchlets |
Absent or rarely present |
Absent |
Absent |
|
Lenticular thickening |
Absent |
Often or occasionally present |
Present |
|
Size of tetrasporangia (μm) |
– |
50 × 25 |
12 × 58 |
|
References |
Chiang 1997; the present study |
||
The second one is also referred to the sect. Spinuligerae and formed the same clade as H. cenomyce (Fig. 4). The genetic distance between both sequences within the clade was calculated as 0.0–0.1%. In general, the presence or absence of medullary lenticular thickenings is considered as one of the useful taxonomic characters in Hypnea (Tseng 1984; Chiang 1997; Xia and Wang 1997; Geraldino et al. 2010). It has thickenings in medullary cell walls like Hypnea cenomyce (Chiang 1997; Yoshida 1998) (Table 1) and is distinguished from other Korean Hypnea species, such as H. boergesenii, H. cornuta, H. flexicaulis, H. japonica, H. saidana, and H. spinella, by the presence of this feature. H. flava forming its sister clade is distinguished from H. cenomyce by epiphytic habitat and presence of anastomoses (Nauer et al. 2016, see table 2). In addition to the presence of lenticular thickenings in the medullary cell walls, this Korean alga is characterized by somewhat entangled thallus at the basal part, percurrent axis, and short spine-like branchlets densely covering axis.
The third alga, which forms a species group corresponding to the sect. Pulvinatae, nests in the same clade as H. nidulans (no intraspecific divergence) (Fig. 4). According to Tanaka (1941), H. nidulans is loosely entangled, and tetrasporangial sori are saddle-shaped, while H. pannosa has densely entangled plants and tetrasporangial sori usually growing on one side of the branchlets, then gradually completely surrounding the branchlets. Dawson (1954) distinguished H. pannosa from H. nidulans by the small size and compact tufts of the former species, but he reduced H. nidulans to the synonymy of H. pannosa based on an examination of the type specimens of both species (Dawson 1961). Later, however, Silva et al. (1996) retained it as a separate species. This is currently accepted (Guiry and Guiry 2018) and is also supported by the present study. H. nidulans appears to be distinguished from those species forming a sister group, such as H. japonica, H. caespitosa, H. pannosa, and H. viridis, by occasionally epiphytic habitat rather than epilithic habitat of low mat-forming growth, as based on the present observation. Percurrent erect main axes, with dense lateral branchlets, also distinguish H. nidulans from those species. This is a feature of the sect. Virgatae rather than the sect. Pulvinatae.
Conclusions
In general, the value of interspecific divergence in the Gigartinales varies from 2.8 to 16.5% (Hommersand et al. 1994; Kato et al. 2009). The interspecific divergence range within the genus Hypnea was calculated as 0.6–7.6% in the present study. This indicates that the genetic distance of 0.0–0.1% between both sequences within each clade formed by the three Korean species is intraspecific within the genus. Based on these morphological and molecular analyses, these Korean Hypnea species are identified as H. yamadae, H. cenomyce, and H. nidulans. This is the first record of these Hypnea species in Korea.