Background
Barbus altianalis also known as the Ripon Barbel is a freshwater cyprinid inhabiting the lacustrine-riverine system of Lakes Edward and Upper Victoria Nile in East Africa (Greenwood 1966). It is a high-value species and a cherished delicacy in the East African region. Its successful induced spawning by Rutaisire et al. (2015) was in response to the drastically declining catches in the region (Ondhoro et al. 2016; Rutaisire et al. 2015). The successful spawning triggered more research activities in technologies aimed at improving its availability for commercial production. For instance, the optimal spawning conditions and its larval growth performance has been improved to ensure mass seed production for distribution (Aruho 2017). However, during the improvement process of spawning, the appropriate size for spawning was found to be an important aspect required for inclusion in spawning protocol. The size at maturity is the size at which 50% of the fish in a given class size is sexually mature (Booth 1997). Length and weight are the most commonly used mean indices for estimating the size at which 50% of the fish become mature in a given class size (Hossain et al. 2012; Lambert et al. 2003). The size at maturity in fish is determined based on gonadal maturation and developmental patterns in a given species (Brown-Peterson et al. 2011; Lambert et al. 2003). Fish grows through egg-larval, juvenile, and mature ontogenetic phases which are genetically and environmentally controlled (Gunnarsson et al. 2006; Nickolskii 1969). Transformation from immature to sexually mature phases in teleosts is a critical developing period when the fish enters the reproductive cycle (Brown-Peterson et al. 2011). Thus, the transition from juvenile to sexual maturity in both females and males occurs when appropriate environmental and biological cues trigger the initiation and transformation for the first time of pre-vitellogenic oocytes and spermatogonia into vitellogenic oocytes and spermatozoa respectively. The attainment of size at maturity in the same species may vary with changing environmental conditions over time and in the same or different geographical locations (Cao et al. 2009; Lambert et al. 2003). The size at maturity has been determined for many teleosts and is regarded as a remarkable factor of reproductive potential that has been widely used in fisheries to control and regulate fish exploitation for sustainable conservation. Its use has apparently been extended into studies for domestication of fish species, guiding culturalists to identify sexually mature and ripe individuals for induced spawning (Aruho et al. 2013; Rutaisire and Booth 2004). Identification of suitable candidates for induced spawning requires adequate knowledge of the size of the fish with high chances of oocytes that have reached ripening stage ready for ovulation (Basiita et al. 2011; Rutaisire et al. 2015).
Although induced spawning was successfully achieved for B. atlianalis (Rutaisire et al. 2015), the appropriate size at sexual maturity was never determined. Any fish ranging from 100 to 7000 g could be used for spawning as long as it had milt or eggs and this is still the trend. However, this raised questions of conservational size and often heightened a potential conflict between fisheries, law enforcers, and breeding scientists as this was seen as a bad habit of encouraging fishers to catch undersized fish. Apparently, it is difficult to state the appropriate size of wild fish for inclusion in the breeding protocol of B. altianalis. In spite of the successful larval weaning and juvenile growth experiments that have been conducted to improve survival and growth of B. altianalis (Aruho 2017), the selection of an appropriate size for breeding is still an important requirement and this is still based on the wild source. This is because there has not been a reported breakthrough of the completion of the breeding cycle of B. altianalis in captivity. Rutaisire et al. (2015) recommended a semi-natural method as one of the propagation methods in which the brood fish could be collected, induced, and left to spawn naturally in ponds. However, the collection of wild species for acclimatization, conditioning, and subsequent domestication requires known length at sexual maturity. Knowledge of size at sexual maturity is an important reproductive aspect that was crucial in defining and integrating the right size of brood fish in artificial spawning protocol of B. altianalis. Thus this study focused on determination of appropriate size (length and weight) at maturity of B. altianalis from two populations of Lake Edward and Upper Victoria Nile to guide in selection of right fish size for induction during artificial spawning.
Methods
B. altianalis samples were collected from Lake Edward (S00.08835, E029.76159; S00.13530, E029.86539) and Upper Victoria Nile (33° 05′ E, 0° 35′ N; 33° 05′ E, 0° 45′ N) between February and April 2015. Samples from Upper Victoria Nile were collected using both hooks and nets. The hooks were of size 9–7 (14–13 mm hook gape size, respectively) mounted on long lines in a fleet of 100–500 m in length and baited with tilapia fingerlings. The fishing nets were used for catching fish in both Upper Victoria Nile and Lake Edward. A fleet of nets each of ply 36, 26 m in width and 90 m deep (for Lake Edward) and 25 m in width and 10 m deep (for Upper Victoria River), with mesh size ranging from 0.5 to 10 inc. were joined together. However, the nets in Lake Edward were not fixed but left to drift in the fishing ground (this is the commonest method used by fishermen for catching B. altianalis in Lake Edward). The nets were set for 12 h at night and for three consecutive days. However, our experimental nets could not catch enough specimens and were therefore supplemented by purchasing all the landed fish on the sampling day. In total, 220 fish were captured with experimental nets from Lake Edward but 303 were purchased, while 200 fish were caught with experimental nets in Upper Victoria Nile but 360 fish were purchase at the landing sites. The total weight (g) was recorded to the nearest 0.1 g and standard length (SL), fork length (FL), and total length (TL) were measured using a calibrated fish measuring board to the nearest 0.1 mm. Fish was dissected to obtain the gonads that were classified into maturity stages based on modifications from Brown-Peterson et al. (2011) classification (Table 1 and Table 4). To validate the stages, small portions of gonads for each fish were immediately preserved in Bouin’s solution for further histological analysis. The preserved samples were taken to the College of Veterinary Medicine, Animal Resources and Bio-security (COVAB) histology laboratory at Makerere University and were processed based on standard histological procedures by Bancroft and Gamble (2002). Tissue subsections of 5 μm of the preserved gonads were dehydrated in different alcohol concentrations in an automatic tissue processor, embedded in wax, further sectioned with a microtome, mounted, rehydrated, and stained using Gill’s hematoxylin and eosin (H&E) and/or Masson’s trichome (MT). The sections were then examined using a light microscope (model Leica DM 500, Made by Microsystems Switzerland Ltd) for identification of oocyte and spermatogenesis stages. The diameter of oocytes and spermatogenic cells were measured by sub sampling at least 5 oocytes for each type in each egg (in 17–25 ovaries from each lake) and at least 30 spermatogenic cells for each type in each testis (in 12–17 testes from each lake) under a light microscope with a calibrated stage micrometer in the eyepiece lenses. For the oocytes, three planes of oocytes and their nuclei diameters were measured and an average size was recorded. The number of nucleoli was counted for each nucleus and recorded.
The differences in sex ratios of fish from each natural water body were estimated by use of a chi-squire method to determine if the ratios were significantly different from the hypothetical 1:1 female to male ratio. Differences in oocyte and spermatogenic cell sizes between fish from Lake Edward and Upper Victoria Nile were determined by student's t-test statistic. The fish were sampled as experimental units from Lake Edward and Upper River Nile using SPSS statistical software (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp, 2013). The length at which 50% (L50) of individuals in a class size were sexually mature was estimated from the ratio of the coefficients of a binary logistic regression of length and maturity level (mature; immature individuals). These coefficients (α and β) of the binary regression were estimated by Excel-solver statistical program in Excel micro software (Windows 2010). The length L50 = Alpha (α)/Beta (β), where α and β are the coefficients of a two-parameter non-linear model (Booth 1997) obtained by stabilizing the coefficients and fitting the logistic ogive curve using Excel-solver.
The two-parameter logistic ogive was described by the non-linear equation:
where PL is the predicted proportion of mature fish at length of the fish L, α and β were coefficients of the parameter model. All individuals in developing phase (stage II) and above (stages II, IV, and V) were taken as mature individuals (Brown-Peterson et al. 2011). A regression between length and weight was run using Excel Microsoft to predict the corresponding weight at L50 for SL, FL, and TL (cm). Comparisons of coefficient b of power equations obtained were based on Froese (2006). To obtain the size frequency distributions (number of sampled fish in each size class) for each size class for the collected data from each lake, individual sizes of fish were grouped in intervals of 5 units using a pivot table in Microsoft Excel. A graph of individual sizes was plotted against the class intervals.Results
A total of 523 specimens from Lake Edward were collected. Females were 314 while the males were 209, constituting a female to male ratio of 1:0.64 which was statistically different from the hypothetical 1:1(χ2 = 18.00, df = 1, P < 0.001). Comparatively, there were 560 fish samples from Upper Victoria Nile. Females were 360 and males were 200 constituting a ratio of 1:0.56. This ratio was significantly deviating from the hypothetical 1:1 female to male ratio (χ2 = 45.71, df = 1, P < 0.001).
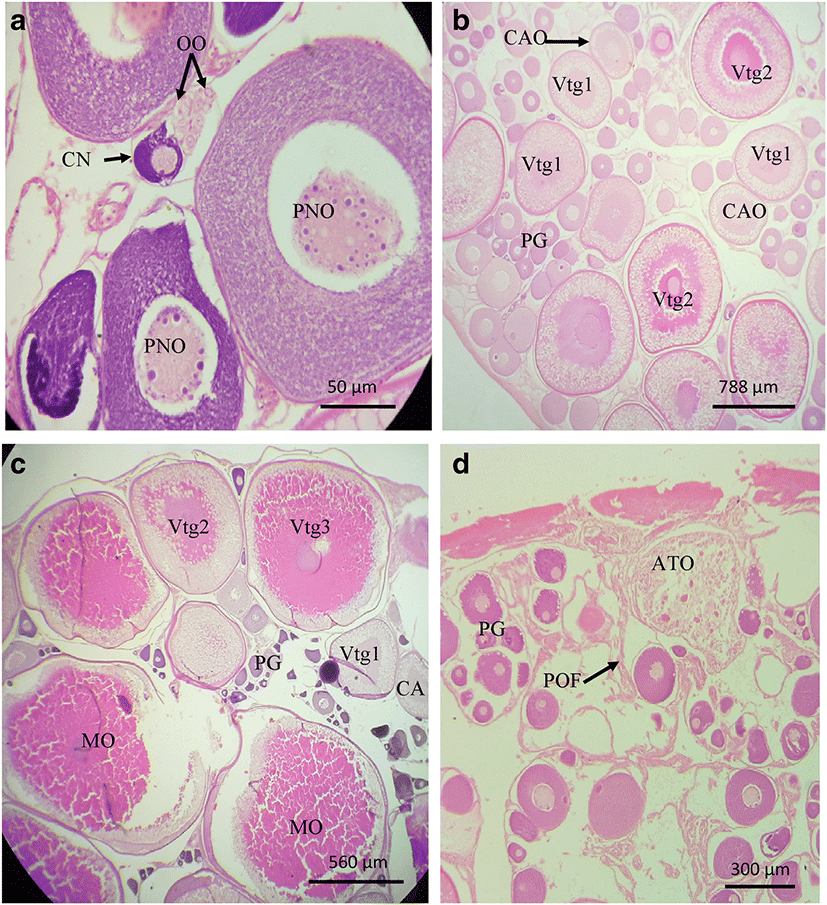
Stage I was dominated by the oogonia and primary growth oocytes which included chromatin nucleolar oocytes and the perinuclear oocytes (Fig. 1a). Macroscopically, the female gonads in stage I were thread-like and transparent (Table 1). In stage II, the primary oocytes developed into cortical alveolar oocytes that later transformed into primary and secondary vitellogenic oocytes (Fig. 1b). Macroscopically, gonads in stage II begun to show minute speckles which later became large and began to fill up the gonad. The gonad became pale yellow as the primary and secondary yolk vesicles began to fill up the gonads (Table 1). In stage III, the secondary vitellogenic oocytes were transformed into tertiary vitellogenic oocytes and mature oocytes. Microscopically, all types of oocytes at this stage were present in the monthly samples indicating batch characteristic development (Fig. 1c). Macroscopically, the gonads swell in size and they became yellowish in color with off-white and yellow egg batches (Table 1, stage III). Stage IV had some post ovulatory follicles (POFs), atretic oocytes, and sometimes very few remaining oocytes (Fig. 1d). The gonad became regressed in size and had relatively bloody looking vessels (Table 1, stage IV). In stage V, only the oogonia and other primary growth oocytes were proliferating. This stage was distinguished from the virgin females by the presence of melano macrophage centers, some increased bloody vessels, old POFs, and atresia of most of vitellogenic stages. The gonad was further reduced but looked dark reddish and not transparent (Table 1, stage V).
There were no significant differences in oocyte sizes for each oocyte type between fish from Lake Edward and those from River Nile (P > 0.5) (Tables 2 and 3).
In stage I, only the primary spermatogonia were present. The testis was tiny, strand-like, and difficult to differentiate from that of the female. In stage II, spermatogonia developed into primary spermatocytes and secondary spermatocytes (Fig. 2a). In stage II, however, few of the secondary spermatocytes had transformed into spermatids and into spermatozoa. The gonads looked off-white with serrations or appearance of lobulations (Table 4). In late stages for stage II, a number of spermertocytes transforming into spermatids and spermatozoa increased. Stage III was characterized by the presence of all spermatogenic cells dominated by the spermatozoa (Fig. 2b). The gonads were bigger than other stages with off-white coloration (Table 4). In stage IV, the number of sperms reduced tremendously living large empty spaces, though there was still presence of all other spermatogenic cells. The gonads were bloody looking. Stage V was characterized by the presence of many spermatogonia with very few residual spermatozoa only.
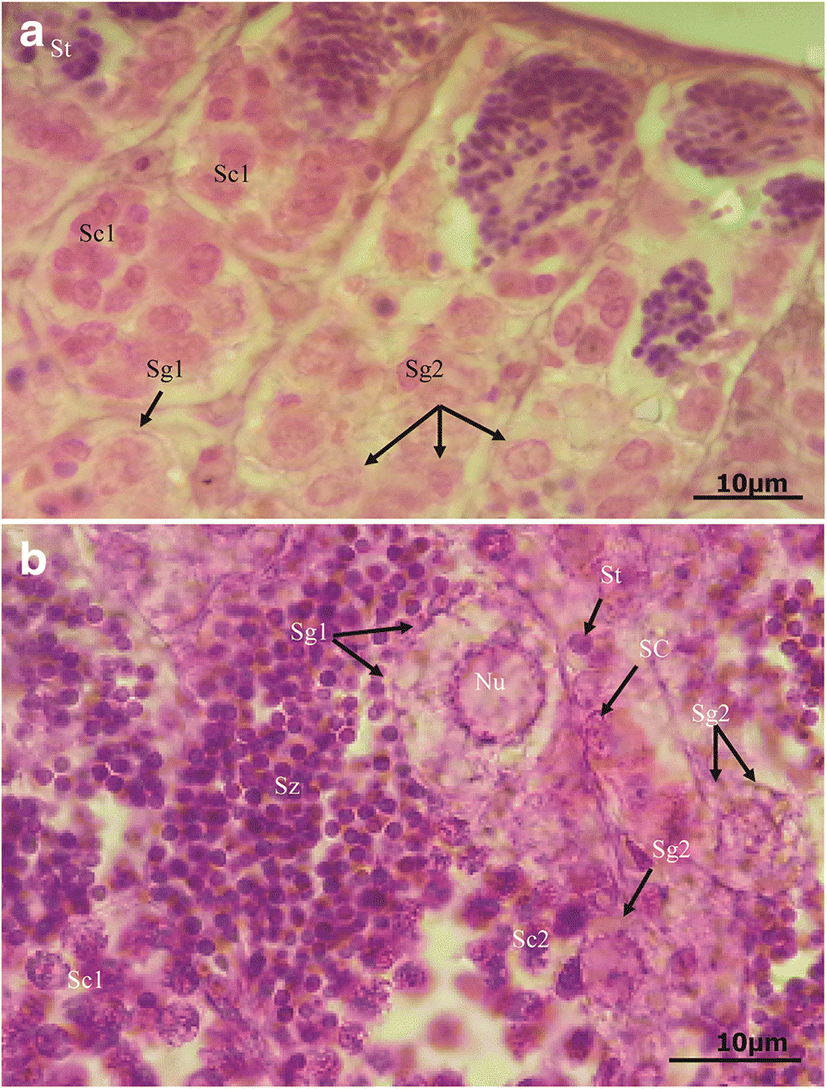
Significant differences in sizes were observed in spermatogonia, spermatocytes, and spermatids between the individuals from Lake Edward and those from Upper Victoria Nile (Table 5). However, no significant differences were observed with spermatozoa between the two populations (P > 0.05). All the spermatogenic cells of males from the Upper Victoria Nile were slightly bigger than those from Lake Edward.
Different subscripts a, b across rows indicate significant differences
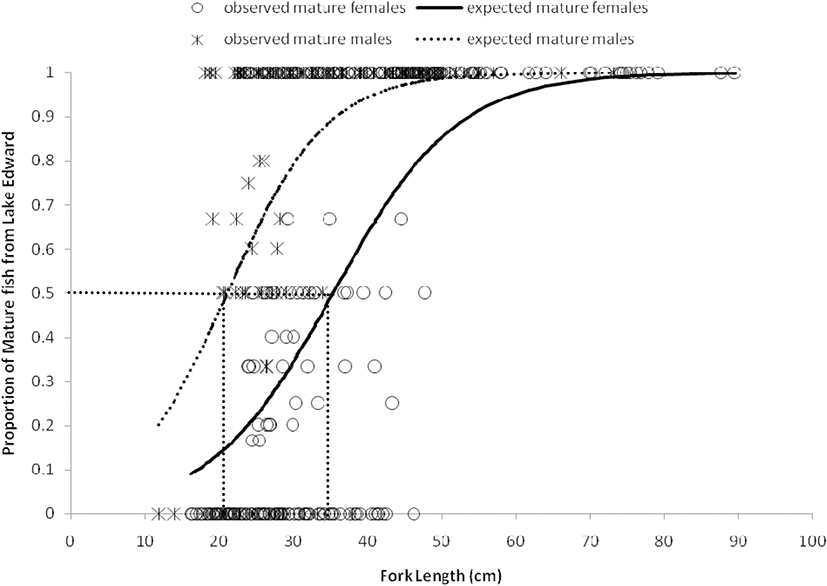
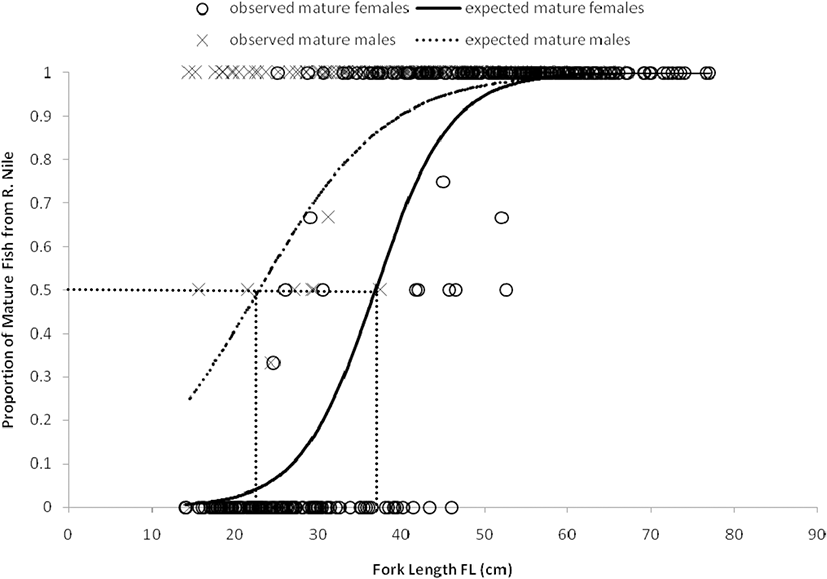
Females and males from Lake Edward reached their sexual maturity at an earlier stage (smaller size) than those from Upper Victoria Nile. Length at maturity L50 for females from Lake Edward and the Upper Victoria Nile were attained at 35.4 cm FL (Fig. 3) and 36.9 cm FL (Fig. 4) respectively and were all found in the same class size of 35–39.9 cm FL. Similarly, the L50 for males from Lake Edward and Upper Victoria Nile were attained in the same class size of 20–24.9 cm FL. Males in Lake Edward attained their maturity at 21.1 cm FL (Fig. 3) while those from River Nile attained their L50 at 22.9 cm FL (Fig. 4). Generally, males from both water bodies attained their L50 much earlier (smaller size) than the females. The L50 at standard length SL and total length TL are also provided in Table 6. The smallest mature female caught from Lake Edward was 22.6 cm FL while the smallest mature male was 18.2 cm FL. The biggest female caught from Lake Edward was 89.5 cm FL (100 TL) while the biggest male was 73.2 cm FL (82.2 cm TL). All males above 37.9 cm FL were mature and all females above 47.7 cm FL were mature. Smallest mature female from Upper Victoria Nile was 24.6 cm FL while the biggest fish caught was 77.0 cm FL (83.0 cm TL). The smallest male caught was 14.5 cm FL while the biggest male caught was 66.2 cm FL (70 cm TL). All females above 52.7 cm FL were mature and all males above 37.6 cm FL were mature.
There was a strong and positive correlation between length and weight (r ≥ 0.90) for both individuals from Lake Edward and Upper Victoria Nile (Table 6). The weights corresponding to L50 (SL, FL, and TL) were estimated by the power equations at each length (Table 6). The power equation value of “b” was ≥ 3.100 for both females and males from Lake Edward and Upper Victoria Nile which was significantly higher than the value of b = 3, as determined by Froese (2006) (Table 6).
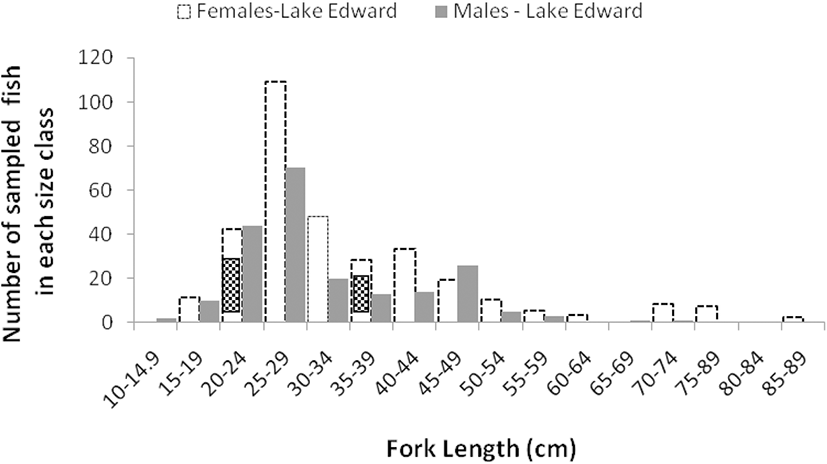
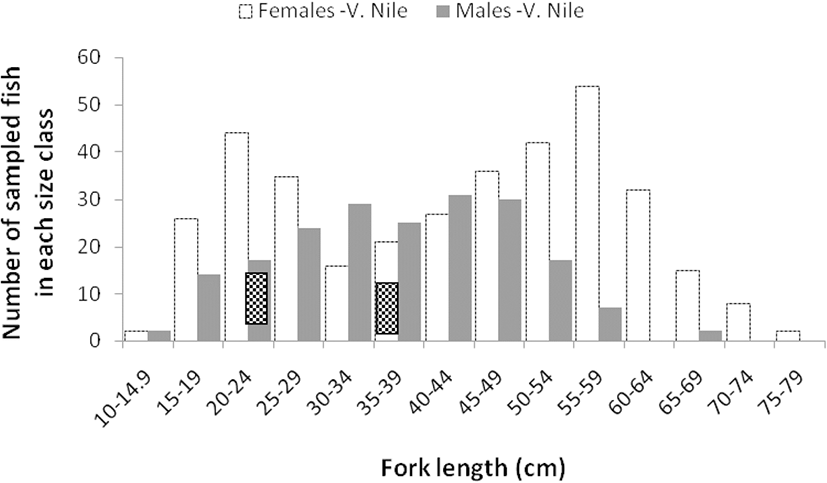
Size frequency distributions of females and males in Lake Edward followed the same pattern, with the highest number of fish recorded in 25–29.9 cm FL class size for both sexes and gradually declining in other class sizes (Fig. 5). The variations shown for males and females from Upper Victoria Nile did not follow a same pattern as those from Lake Edward (Fig. 6). The males were more frequent in class size of 30–34.9 cm to 45–49.9 cm FL and there after they declined. The highest peak for females was obtained in a class size of 55–59.9 cm FL. The males from both water bodies were conspicuously absent or very few in upper class sizes above 60–64.5 cm FL compared to females. For the females from Upper Victoria Nile, high frequencies were noted in upper class sizes while males almost disappeared (Fig. 6).
Discussion
The availability of quality and sufficient seed is the cornerstone for the successful commercialization of domesticated fish species (George et al. 2010; Mair 2002). The seed is a reflection of a healthy and quality broodstock that will eventually determine the availability of quality seed for commercial production of the species. In a number of cultured species, the brood fish is an integral process of a well-planned seed production system (Bondad-Reantaso 2007). One of the key characteristics of the spawning technology is the appropriate size and the source as well as availability of both broodstock females and males. The wild sources are still the most significant sources of the broodstock for newly domesticated fish and grading up of genetically depressed captive stocks. In this study, sex distribution from Lake Edward and Upper Victoria Nile were found to be the same, thus the sex ratios of females to males were similar in both water bodies. The ratios were skewed toward more females. Skewed sex ratios could suggest some migratory activity related to breeding seasons (Mahmood et al. 2011), a shorter life span for males, a natural intrinsic characteristic for biased sexes, or environmental variations (Baroiller et al. 2009; Vandeputte and Quillet 2012). In spite of the fact that samples were peaked in a dry month of February and wet months of March and April, a biased sex ratio was observed. Therefore, it seems that sex ratio bias toward females is genetically predisposed. Nevertheless, the occurrence of males and females at the same sampling points suggested that it is much easier for the farmer to collect both males and females for breeding (Aruho et al. 2013).
This study observed that the general developmental and maturation process in B. altianalis was similar to that described in other cyprinid species (Booth and Weyl 2000; Lone and Hussain 2009; Maack and Segner 2003; Smith and Walker 2004). However, special specific processes including the color and size of gonads, oocytes, and spermatogenic cells, the location of the nucleus, and number of nuclei may vary for each species and were inevitably described to ensure a clear guided description for identification of classification staging as suggested by Brown-Peterson et al. (2011). Although size variations were only noted with spermatogonia, spermatocytes, and spermatids among the two populations, the final spermatozoa sizes emerging from the cysts during spermiation process were the same, attributing the variations to differences in cytoplasmic volume. As observed with spermatozoa, there were no differences in oocyte sizes between the two populations from both water bodies. However, a study by Muwanika et al. (2012) suggested that morphological and genetical differences occurred between the two population clusters but did not identify them as different morphs or species and recommended further investigations because the sample size was small and required robust genetic methods. Based on the current results, it seems the two populations remain the same with less divergent strategies or rather still “primitive” in its evolutionary process. Slight differences observed in cytoplasic volume between the two populations could be linked to localized environmental conditions for each water body. Localized changes in the water environment, preferential food requirements, or fishing pressure may account for the observed differences (Lam 1983). Genetic studies are required to help ascertain if the differences between these groups exist. However, since no significant differences in oocytes and spermatozoa were observed between the two populations, the same classification (staging) was maintained for B. altianalis from both water bodies for description of length at maturity. This is important for guiding farmers and other fisheries scientists in staging B. altianalis gonads.
Results from this study showed that the size at maturity L50 for B. altianalis males from Upper Victoria Nile and from Lake Edward were obtained in the same class size as was for females. This finding further suggests that in spite of two water bodies being separated or having no close connection the species has not separated into other morphs or forms. Despite the fact that they were in the same class size, the L50 values were not exactly of the same length. In species such as Oreochromis niloticus (Duponchelle and Panfili 1998), Peritalbagrus fulvidraco (Cao et al. 2009), and Hippoglossoides platessoides (Morgan and Colbourne 1999), the variations observed in L50 in their population cohorts in different sampling areas were attributed to fishing pressure and environmental differences (phenotypic plasticity) other than genetic differences. Therefore, the minor differences in L50 observed in B. altianalis were probably linked to environmental differences other than genetic variations. It seems B. altianalis still retains very primitive traits with longer evolutionary characteristics and thus fish from Lake Edward and Upper Victoria Nile could easily be crossbred to obtain the best traits possible for culture. The study also revealed that males matured much earlier than females. Early maturation of males ensures that all eggs produced by females are fertilized during spawning (Aruho 2017).
The selection of an appropriate broodfish for spawning is largely linked to knowledge of maturation size and takes into cognizance of conservation of the wild stocks to avoid over exploitation. For instance size frequency distribution noted in this study suggests that the 50% of the sampled population (L50) for B. altianalis males from Lake Edward and Upper Victoria Nile obtained in a class size of 20–24.9 cm FL (135.8 g–178.7 g) was smaller than the minimum legally recommended sizes of 30–34.9 cm FL (about 500 g). The recommended fishing nets for catching this size is ≥ 4.5 in. mesh size (amendment Fish Act, 1951). This implied that any size below 30–34.9 cm FL is not legally acceptable for harvesting as broodfish. Secondly, in spite of the fact that the noted L50 could appropriately be collected for spawning, the weight is rather small for handling in order to obtain milt and also not appropriate for selection of good breeds. Hence, this study suggests that males for breeding fish could appropriately be picked from class size of ≥ 30–34.9 cm FL (417–675 g) for males and ≥ 35–39.9 cm FL (700–726.4 g) for females.
The study showed that males and females from Lake Edward were more frequent in a class size of 25–29.9 cm FL than in other class sizes suggesting an improved enforcement effort of fishing regulations for the right net sizes on the lake. The reduction thereafter implied fishing effort or activity. The pattern of females and males in Upper Victoria Nile did not suggest a relationship with fishing activity. The frequency of females was notably high in the class sizes of 15–19.9 to 25–29.9 cm FL and from 40–44.9 to 60–64.9 cm FL, while the male frequencies were high between 30–34.9 and 45–49.9 cm FL. This implied that there was little fishing pressure. There is a reported regular interruption of power generating activities at Kira and Bujagali power Dams that bars fishers from fishing daily and some portions are restricted and inaccessible by fishermen (personal communication with fishers; David Odong, head of fishermen at Kiira landing site). This could further explain why the L50 values for fish from Upper Victoria Nile were slightly higher than those from Lake Edward for both females and males. Hence, sustained fishing pressure of B. altinalis is more likely to influence evolutionary changes in L50 for Lake Edward individuals first before those from Upper Victoria Nile. Sustained selective fishing pressure has been suggested to cause rapid changes in evolution of maturation schedules in many fish species (Audzijonyte et al. 2013; Hunter et al. 2015; Law 2000). The frequency distribution and the L50 observed in B. altianalis from this study showed that very few or no males were caught above 65 cm FL. In some fish species, shorter male life spans were associated with aggressive behavior of males making them vulnerable to enemies during feeding or mating (King et al. 2013; Reichard et al. 2014). However, further investigations are necessary to ascertain if there is any behavior that makes B. altianalis susceptible to predators or fishing.
The coefficient “b” of the power curves obtained for the weight–length relationship of the sampled population suggested that natural growth rates were similar for both populations from Lake Edward and Upper Victoria Nile because all the “b” values were in the same range of 3.126–3.164 for both males and females. This was also reported by Ondhoro et al. (2016) for the same species. These values were significantly higher than b = 3 (Froese 2006) implying that the fish girth increased much faster than the length (Froese 2006; Karachle and Stergiou 2012). Therefore, B. altianalis from Lake Edward and Upper Victoria Nile were accurately grouped under species with positively allometric growth. The weight–length power equation had a strong r2 of > 97%; hence, the weights at each L50 were and can be accurately estimated using the power equations obtained in this study.
The farm records for the cultured B. altianalis (Additional file 1) also indicated that males matured at smaller sizes than females as in the wild populations. In spite of the fact that L50 was not determined for the farm record samples because of the very small numbers, the fewer number of fish from the records show that both females and males matured at smaller sizes compared to their counterparts in the wild. This is common with many cultured species, and it is a strategy by the fish to ensure earlier production of offsprings under confined and or unpredictable environmental conditions (Brummett 1995; Longalong et al. 1999). It is imperative though to use bigger males (≥ 30–34.9 cm FL) as earlier suggested, than the indicated sizes for easier selection of good broodstock. However, a thorough experimental study is recommended for determination of appropriate values of L50 in ponds with appropriate feed.
Conclusions
Evidence obtained from sex ratios, L50, power equations, and germ cell sizes in this study indicated that B. altianalis males and females from Lake Edward were similar to those from Upper Victoria Nile; hence, there are no clear evolutionary lines detected for this species and the species has remained more or less primitive. The species could therefore be crossbred where applicable for the best cultured traits. The length at which 50% individuals were mature was 20–24.9 cm FL for males and 35–39.9 cm FL for females. But because of the need to strict adherence to fishing regulations and conservation of the wild resources as well as the need to have selection of good broodfish, the study recommends selecting individuals from 30 to 34.9 cm FL for males and 35 to 39.9 cm FL for females to be included in the spawning protocol. These individuals could be picked from the same areas for breeding and during the periods when breeding fish are prominent (Rutaisire et al. 2015). Because the famed fish attained smaller sizes at maturity than their wild counterparts, this may point to the fact that more growth study experiments with appropriate conditions and diet could be required to improve the growth performance of B. altianalis.