Background
Collagen is a major structural protein that is widely distributed in animal connective tissues. The primary structure of collagen is unique as it contains a glycine-rich repeat sequence (Gly-X-Y), in which prolyl and hydroxyprolyl residues at the X and Y positions determine the triple helical secondary structure (Gordon and Hahn, 2010; Ramshaw et al., 1998). Collagen is widely used in the food, cosmetic, biomedical, and pharmaceutical industries. Commercial sources of collagen are mainly derived from mammals such as cows and pigs. Marine collagen is advantageous over mammalian collagen because (i) marine animals are not affected by infectious diseases such as avian influenza, bovine spongiform encephalopathy (BSE), transmissible spongiform encephalopathy (TSE), and foot and mouth disease (FMD) observed in pigs and cattle, (ii) the consumption of marine collagen is acceptable to people with religious restrictions, and (iii) it has a lower thermal denaturation temperature than collagen of terrestrial animals (Nagai et al., 1999; Nagai et al., 2010; Senaratne et al., 2006), which is conducive for assimilation by the human digestive system.
In 2002, the first full-cycle aquaculture of Pacific bluefin tuna (PBT) was successfully performed at Kindai University, Japan (Sawada et al., 2005). Currently, more than 40,000 cultured juveniles are available from the bioventure company, A-Marine Kindai (Wakayama, Japan). This increased supply of bluefin tuna has triggered research into the effective use of the unused parts of the tuna, such as its skin and organs, to avoid environmental pollution and to promote economic sufficiency. Therefore, we have focused on PBT skin as a collagen-rich underused resource for functional food. Previously, we reported that dietary PBT skin protein and collagen hydrolysis exerts hepato-protective action in CCl4-intoxicated mice (Tanaka et al., 2012). In addition, the collagen from PBT, but not from salmon, mackerel, and carp, also reduced HepG2 and HeLa cell growth in a dose-dependent manner, suggesting the existence of a PBT skin collagen-specific primary structure and/or higher-order structural conformation (Han et al., 2011). However, little is known about characteristic feature and structural information of isolated PBT skin collagen.
Studies on the early life history of PBT, which addressed the morphological changes (Kaji et al. 1996; Miyashita et al. 2001), chemical content, enzyme activities (Takii et al. 1997), and the development of the digestive system during PBT embryogenesis (Miyashita et al. 1998), have provided information required for the development of mass seeding techniques. However, laboratory-reared PBT suffer high mortality during the rapid somatic growth stage in their early life (Sawada et al. 2005; Tanaka et al. 2007). For example, PBT possesses very sensitive skin, which renders its handling difficult during rearing of this species. Over 40% PBT juveniles die of skin injuries that are incurred during transportation with hand nets in the first week of transfer of these land-based farmed juveniles to open net cages (Ishibashi et al., 2009). Therefore, it is important to understand the property of type I collagen, which is a major component of PBT skin.
In this study, we isolated skin collagen from PBT and characterized certain properties.
Methods
Calf and salmon skin type I collagens were purchased from Wako Pure Chemicals (Osaka, Japan). All chemicals used in this study were of the highest purity available.
PBT (24–32 days after hatching) was obtained in an unfrozen state at 4 °C within 24 h after catching the tuna from culture fields of Aquaculture Research Institute, Uragami Station, Kindai University, Japan. The skin was dissected from the body and stored at − 20 °C. Bluefin tuna skin collagen was isolated using a previously reported procedure (Han et al., 2011) with slight modifications. All steps of the extraction was performed at 4 °C. The skin of PBT without the muscles and scales was cut into small pieces. The pieces were soaked in 0.1 M NaOH for 24 h with stirring. The NaOH solution was changed every 8 h to remove non-collagenous proteins and pigments. The pieces were washed with distilled water until neutral pH was obtained. The pieces were then defatted with methanol/chloroform (2:3) and washed with methanol and distilled water. For extracting collagen, the defatted pieces were stirred in 10 volumes (w/v) of 0.5 M acetic acid for 24 h. Pepsin (3130 U/mg solid; Nacalai Tesque Inc. Kyoto, Japan) was then added to the supernatant (7 μg/L), and the mixture was gently stirred for 48 h. Collagen was precipitated by salting out with 25% (w/v) NaCl and centrifuged at 5000×g for 30 min. The precipitate was dissolved in 0.5 M acetic acid and centrifuged (15,000×g, 60 min). The supernatant was dialyzed with stirring for 24 h against five changes of distilled water and lyophilized. The collagen sample was stored at − 20 °C until further analysis.
SDS-PAGE was performed using the Tris-HCl/glycine buffer system and 7.5% polyacrylamide gel described by Laemmli (1970) using the Tris-HCl/glycine buffer system with a 7.5% resolving gel and 4% stacking gel. The collagen sample was dissolved in sample buffer (0.5 M Tris-HCl, pH 6.8, containing 8% SDS, 30% glycerol, 0.2% bromophenolblue) containing 5% β-mercaptoethanol and then boiled for 5 min. Collagen samples (50 μg/well) were applied to sample wells and electrophoresed. The separated proteins were stained with Coomassie Brilliant Blue R-250. Peptide mapping was performed as described by Yata et al. (2001). The isolated collagens were digested with lysyl endopeptidase (Wako Pure Chemicals, Japan) at an enzyme/substrate ratio of 1:100 (w/w). Peptides generated by the protease digestion were separated by SDS-PAGE using 7.5% gel. The separated proteins and peptide were stained with Coomassie Brilliant Blue R-250.
The collagen sample was hydrolyzed in 6 N HC1 at 110 °C for 24 h. The hydrolysates were analyzed using an L-8800 automated amino acid analyzer (Hitachi High-Technologies, Tokyo, Japan).
As previously reported by Nomura et al. (1996), the denaturation temperature of PBT skin collagen in 0.5 M acetic acid was measured using an Autopol III automatic polarimeter (Rudolph Research Co. Flanders, N J) at 589 nm.
The ultraviolet absorption spectra of collagen were recorded using a spectrophotometer (U-0080D, HITACHI, Japan) from 190 to 400 nm. The isolated collagen was dissolved in 0.5 M acetic acid to obtain a concentration of 0.05% (m/v).
Attenuated total reflection (ATR)-FTIR spectra of collagen was obtained using a Nicolet 6700 FTIR Spectrometer (Thermo Fisher Scientific, USA) equipped with ATR accessory. Spectra were recorded from 4000 to 500 cm− 1 at a data acquisition rate of 0.5 cm− 1 per point.
The cross-reactivity of PBT type I skin collagen with IgG-purified guinea pig antibody against salmon type I collagen was examined using three enzyme-linked immunosorbent assays (ELISA).
ELISA plates were coated with serial dilutions of collagen in phosphate-buffered saline (PBS) to determine the linear range of the sigmoid curve. Next, the plates were blocked with blocking buffer (200 μL) (Blocking One-P, Nacalai Tesque Inc. Kyoto, Japan) for 1 h at room temperature. Horseradish peroxidase (HRP)-labeled antibody against salmon collagen (100 μL) was added at 1/1000 dilution in PBS and incubated for 1 h at room temperature. The specificity for binding with immunoglobulins of salmon collagen antibodies was previously tested using western blot (data not shown). After incubation with 3, 3′, 5, 5′-tetramethylbenzidine (TMB) substrate buffer, absorbance was measured using a plate spectrophotometer at 405 nm.
ELISA plates were coated with 100 μL of 10 μg/mL salmon collagen antibody for 13 h at 4 °C. After blocking, the plates were incubated with serial dilutions of collagen (100 μL) in PBS for 1 h at room temperature. Next, HRP-labeled salmon collagen antibody (100 μL) was added at 1/1000 dilution in PBS and incubated for 1 h at room temperature. The colorimetric method was performed as mentioned above using the TMB substrate.
Serial dilutions of collagen were coated on ELISA plates. After coating, the plate was incubated for 1 h at room temperature (200 μL). Pre-incubated (1 h at room temperature) dilutions of a salmon collagen and HRP-labeled salmon collagen antibody were added and incubated for 1 h at room temperature. The colorimetric method was performed as mentioned above using the TMB substrate.
Results
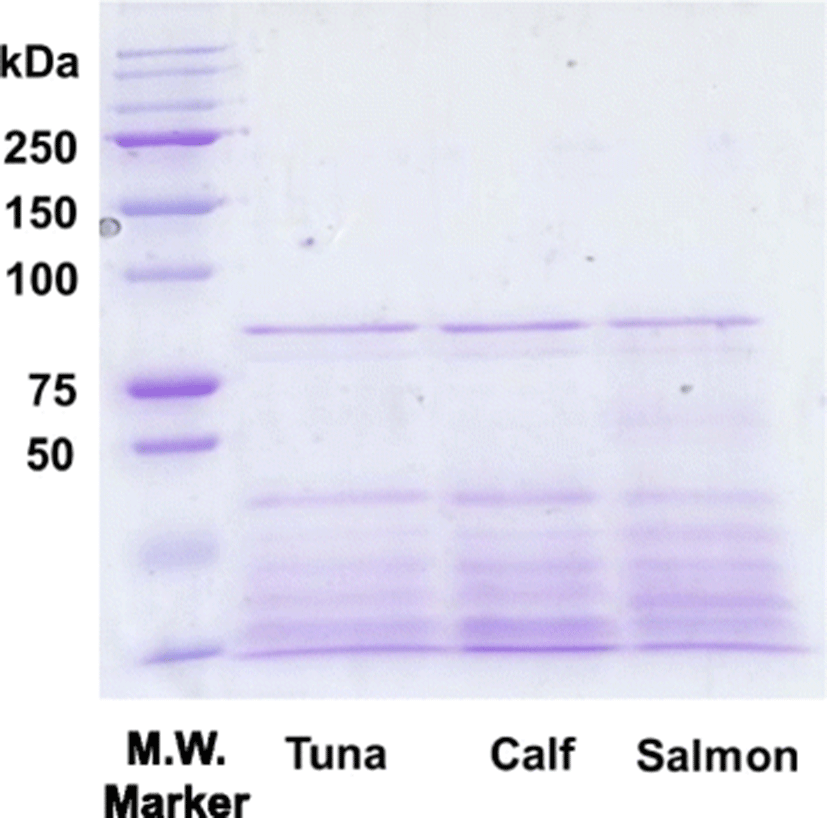
In this study, the acid-soluble skin collagen of PBT was isolated. The final protein recovery rate of the PBT skin collagen was 2.1 g/100 g and the dry yield was 5.4%. The isolated PBT skin collagen was analyzed using SDS-PAGE. The separation pattern shows that PBT skin collagen was composed of two α chains (α1 and α2) and one β chain similar to calf and salmon collagen (Fig. 1). The estimated molecular weights for the α1 and α2 chains were approximately 120 and 112 kDa, respectively, which is similar to previous observations (Nalinanon et al., 2007).
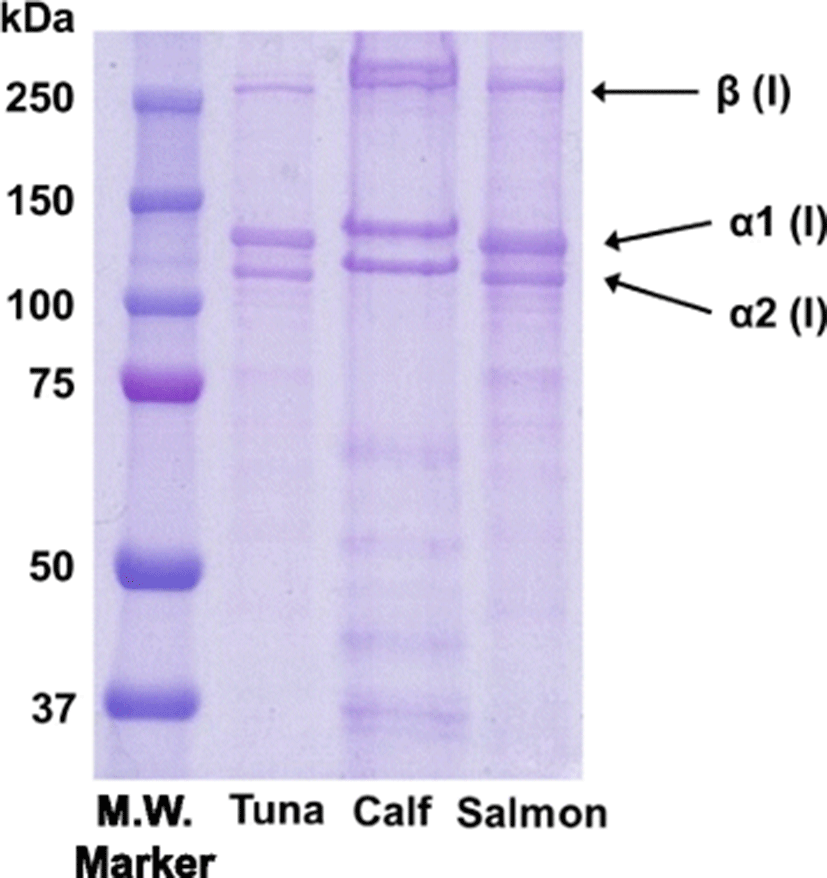
Table 1 shows the amino acid composition of the PBT skin collagen. Glycine was the most abundant amino acid in the PBT skin collagen with a content of 27.58%. This is similar to the glycine content of calf (Giraud-Guille et al., 2000) and salmon skin gelatin (Arnesen and Gildberg, 2007). In addition, PBT skin collagen had high content of proline, alanine, and arginine; however, cysteine and phenylalanine were not detected.
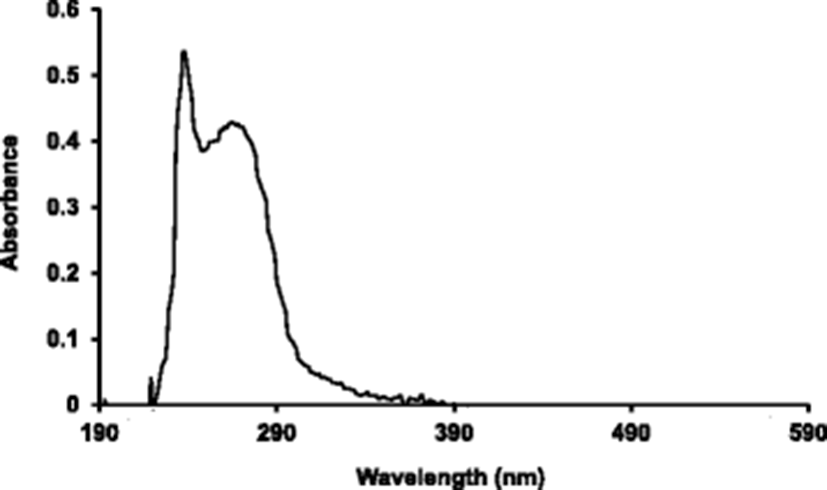
Figure 2 shows the UV-Vis spectra of PBT skin collagens scanned at 190–590 nm. The major peak was observed at 238 nm. There was also a swell distribution between 250 and 280 nm.
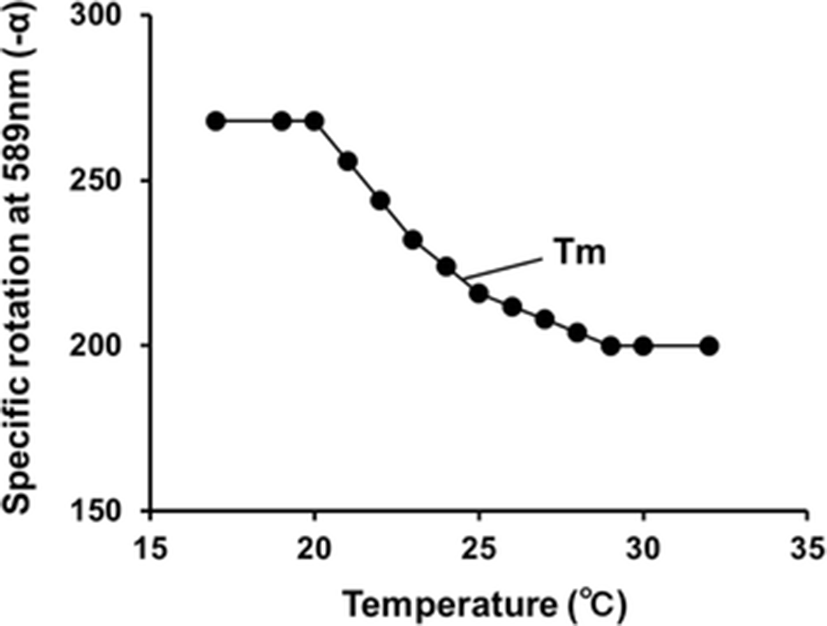
As shown in Fig. 3, the change in optical rotation of PBT skin collagen in solution started at 20 °C and finished at 29 °C. Thus the denaturation temperature (mid-point, Tm) of the PBT skin collagen was estimated as 24.5 °C.
Peptide mapping was performed to compare the primary structure of PBT skin collagen with calf and salmon skin collagen. The electrophoretic patterns of lysyl endopeptidase-digested PBT, calf, and salmon skin collagen were observed on a 7.5% denaturing polyacrylamide gel. As shown in Fig. 4, the electrophoretic pattern of PBT skin collagen was similar to those of calf and salmon skin collagen, indicating that the cleavage site of PBT skin collagen by lysyl endopeptidase was almost identical to those of calf and salmon skin collagen.
To compare the partial sequences and higher-order structure of PBT skin collagen with calf, and salmon skin collagen, direct, sandwich, and inhibition ELISA were established. We examined cross-reactivity between the isolated PBT skin collagen and IgG-purified guinea pig antibody against salmon type I collagen. As shown in Fig. 4, the calibration ranges established using direct, sandwich, and inhibition ELISA were 10–1000, 10–10,000, and 10–100,000 ng/mL, respectively. In the direct ELISA, the PBT skin collagen reacted with the antibody against salmon type I collagen, but the reactivity was almost similar to that of calf collagen (Fig. 5a). In addition, the PBT skin collagen was not detected in sandwich ELISA, suggesting that its epitope structure differed from that of salmon collagen (Fig. 5b). The PBT collagen recognized the antibody in inhibition ELISA, although the reactivity to the antibody was appreciably weaker compared to that of salmon collagen (Fig. 5c). The difference in cross-reactivity of PBT and salmon collagen might reflect the variations in epitope recognition of the antibody.
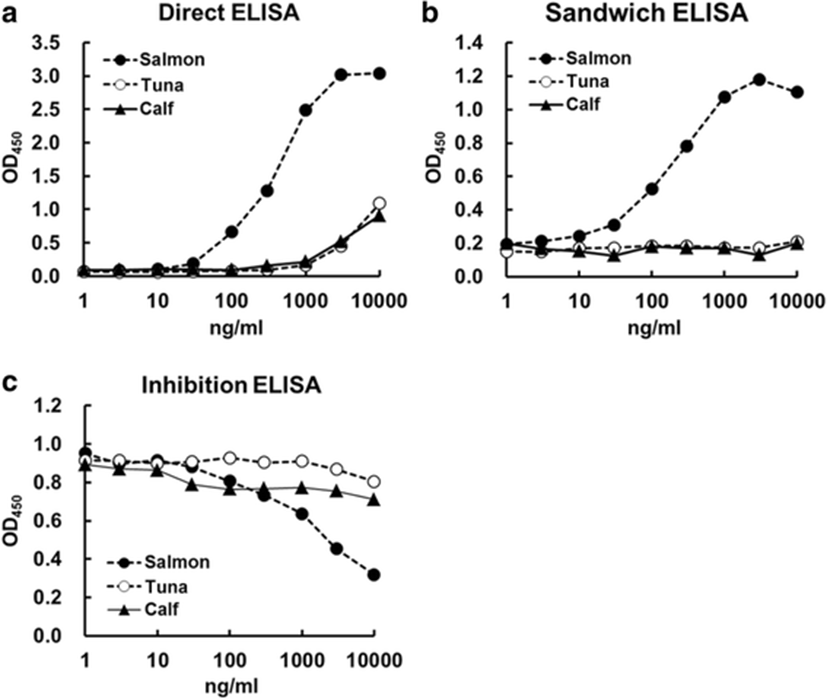
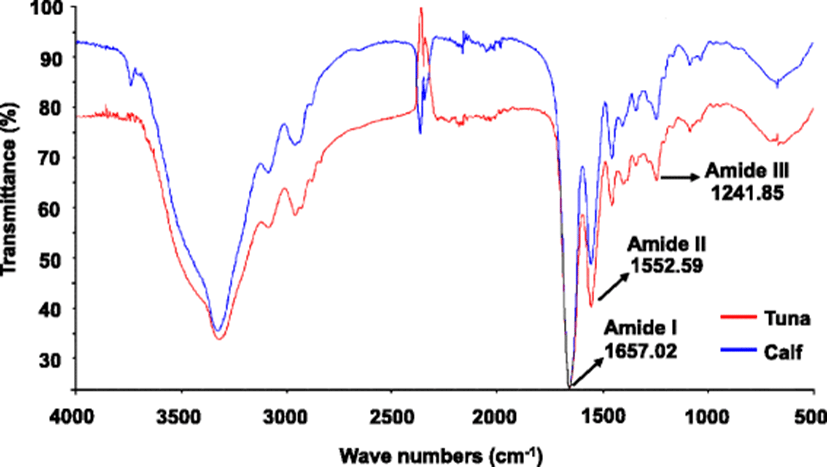
Figure 6 shows the FTIR spectra of PBT and calf skin collagen. The spectra of PBT skin collagen were roughly similar to those of calf collagen. The spectra of PBT dispersions demonstrated a characteristic pattern reflecting the amide I band at 1657 cm− 1, the amide II band at 1553 cm− 1, and amide III band at 1241 cm− 1, derived from C=O stretching, N–H bending vibrations, and C–H stretching (Payne and Veis, 1988), respectively. The amide I band, which is associated with the secondary structure of the protein, and the amide III band demonstrated the existence of a helical structure (Muyonga et al., 2004, 2004). These results suggest the existence of helical arrangements in the extracted PBT collagen.
Discussion
In this study, we isolated and characterized certain properties of PBT skin collagen. The PBT skin collagen was composed of two α chains (α1 and α2) and one β chain. This result is similar to previous reports on collagen characteristics of other fish species (Tan and Chang 2018; Muyonga et al., 2004; Yata et al., 2001). UV-vis and FTIR spectra of PBT skin collagen resembled that of type I collagen reported previously. All these data suggest that the isolated collagen is a typical type I collagen. In the present study, we did not perform proximate analysis of fish skin during the isolation process. The possible differences in the yield obtained during the isolation process between these species are a limitation of this study.
The denaturation temperature of the PBT skin collagen was lower than that of other fish collagen. The thermal denaturation temperature of collagen is related to the proline and hydroxyproline content (Wong, 1989). The Pro and Hyp content in PBT skin type I collagen were 10.5 and 6.4%, respectively; the ratio of Pro to Hyp in PBT is higher than that in salmon (Arnesen and Gildberg, 2007), big eye snapper (Kittiphattanabawon et al., 2005), and skate (Hwang et al., 2007). However, the thermal denaturation temperature of PBT skin collagen was lower than that of salmon (28.7 °C), torafugu, and skate (28.8 °C).
Previous studies have revealed the primary structure of type I and II procollagen α1 chain in some fishes (Saito et al., 2001; Hwang et al., 2006; Zhang et al., 2016). We cloned the cDNA for PBT procollagen α1 (I) (Tanaka et al., 2014) and predicted that the PBT procollagen α1 (I) might contain high numbers of Gly-Gly sequences (Gly-Gly and Gly-Gly-Gly) in the triple-helical region. The number of Gly-Gly sequences in PBT procollagen α1 (I) was 14, whereas the number in zebrafish, rainbow trout, and torafugu were 4, 22, and 11, respectively. Since Gly is the smallest amino acid, the Gly-Gly sequence likely contributes to the partial skew in the triple helix structure and the decrease in thermal stability. While the PBT procollagen α1 (I) contains a high number of Gly-Gly sequence, it is not the highest among fish procollagen α1 (I) reported previously. Thus, further rationalization for the low thermal stability of PBT skin collagen is required. In addition, two Ser residues (1253 and 1270) that play a crucial role in the interactions of the procollagen α chains (Dion and Myers, 1987) were not found in the C-terminal region of the PBT procollagen α1 (I) chain. This indicated that PBT collagen might easily accrue distortion in its protein structure, which might contribute to its low denaturation temperature. PBT possesses delicate skin, which renders handling difficult during rearing this species. The primary structure of the PBT skin collagen could possibly explain the sensitive nature of its skin.
Tryptophan and phenylalanine are not present in the PBT collagen and the tyrosine content was 0.35%. Because, it is generally considered that most proteins that absorb at 280 nm of the UV-Vis spectra contain tyrosine, tryptophan, and phenylalanine, the absorption peak at 280 nm was weak. The major peak at 238 nm was slightly different from the skin collagen of largefin longbarbel (Zhang et al., 2009) at 232 nm and collagen of abalone gastropod muscle (Dong et al., 2012) at 233 nm. These differences might be due to differences in amino acid content between PBT collagen and other collagen.
The electrophoretic patterns of lysyl endopeptidase-digested PBT was similar to those of calf and salmon skin collagen as well as the electrophoretic pattern for acid-soluble collagen. Therefore, the primary structure of PBT skin collagen, including the cleavage site by lysyl endopeptidase, was almost identical to that of calf and salmon skin collagen. However, the cross-reactivity of PBT type I skin collagen with salmon collagen antibody was weak. The difference in cross-reactivity of PBT and salmon collagen might reflect the variations in epitope recognition of the antibody. These results suggest that although the primary structure of collagen type I is highly conserved in animal species, the partial sequences that include the epitope structure differ significantly. An antibody against PBT collagen is required for more accurate characterization of tuna collagen.
Most fish collagens are composed of two α1 and one α2 chains (Gómez-Guillén et al., 2002; Muyonga et al., 2004). Piez (1965) reported that cod skin collagen has three variants of α chains (α1, α2, and α3) that differ in amino acid composition. Subsequently, the α3 chain was identified in collagen of other fish skin. Although the PBT skin collagen may contain the α3 chain, its presence was not determined using ion exchange chromatography in this study. Therefore, further studies are required to elucidate this point.
In the present study, we did not calculate the extraction efficiency of skin collagen halfway during the extraction process. However, this efficiency will be calculated by determining the hydroxyproline content in the sample in our next study. In addition, the proximate analysis of fish skin and the yield during the isolation process was not performed. The differences in the yield obtained during the isolation process between these animal species are a limitation of this study.
In addition, type I collagen has been identified as cross-reactive allergen for fish allergies (Hamada et al., 2001). Although the difference in cross-reactivity of PBT and salmon collagen was showed in this study, Kobayashi et al. (2016) clarified that pooled serum obtained from patients with fish collagen-specific allergies exhibited IgE reactivity to extracts from Atlantic salmon (Salmo salar) and yellowfin tuna (Thunnus albacares) by direct and inhibition ELISA. The cross-reactivity of bluefin tuna collagen with salmon collagen antibody provided information relevant for structural studies. Therefore, epitope recognition by anti-collagen antibody might differ among tuna species. However, further studies are required to understand its structural integrity.
Conclusion
In summary, the PBT skin collagen is composed of two α chains (α1 and α2) and one β chain. The PBT collagen has low denaturation temperature, although it is rich in proline and hydroxyproline. The primary structure of PBT skin collagen was approximately identical to that of calf and salmon skin collagen; however, it varied from the others with respect to epitope recognition of the antibody against salmon type I collagen. Further studies are required for understanding the specific primary or higher-order structure of PBT collagen.