Background
Oysters are suspension feeders that graze phytoplankton in the surrounding water column. This feeding behavior is facilitated by the cilia located on the gill filaments. These filaments generate water currents over the gills, select for food particles from the water column, transport food particles towards the mouth, and reject pseudofeces onto the mantle (Newell and Langdon 1996). Since the velocity field for feed behavior is highly restricted within organism’s microenvironment (Uslu and Pekkan 2016), primary production could be a key factor in determining the productivity of oyster farms.
The present study site is located on the tidal flat of the eastern coast of Wando, which is positioned in narrow waterway between Wando and Gogeum Island. This area was previously well known for Porphyra aquaculture. Annual production in this region accounted for ~ 22% of national production in South Korea; however, an environmental change linked to changing demographic and industrial development means that Porphyra aquaculture has ceased in the tidal flats in Wando (Lee 2006; Han and Cho 2013). As an alternative fishery in the tidal flats, oyster rack culturing was introduced as a pilot project in 2013 (Cho et al. 2013a). Here, we optimized several conditions to achieve the maximum sustainable yield (MSY) in these oyster rack cultures (Han and Cho 2013).
To ensure the successful management of oyster culture, many factors must be considered, but primary production is one of the most vital to achieving the MSY. Since oysters are filter feeders that rely solely on the surrounding environment as an energy source, an accurate estimation of primary production (PP) is essential to evaluate the potential yields in oyster farm (Lee et al. 1991). In this study, we estimate the PP to optimize the cultural practices (e.g., cultural density, nursery technique) that can yield crucial information on the best practices for managing an oyster farm.
Methods
Seawater was sampled monthly at the surface of five sampling sites from June 2011 to December 2012 (Fig. 1) using a Niskin sampler. Water temperature, salinity, and dissolved oxygen (DO) were measured by multiprobe system (YSI 556, YSI, Ohio, USA). Chlorophyll-a content was determined as described by Parson et al. (1984) after filtration of seawater using GF/C filter. Dissolved inorganic nitrogen (DIN) and dissolved inorganic phosphorus (DIP) were determined followed by Korean standard method for marine environment (Fisheries 2013).
To estimate primary production, a numerical model was developed to predict photosynthetic rate from water temperature, solar irradiation, and chlorophyll-a content (Jeong et al. 2009). The model is briefly described below.
Solar irradiance at the sea surface was determined using an LI-1000 datalogger equipped with an LI-190SA quantum sensor (LI-COR, Nebraska, USA; mol m−2 s−1), which was situated on the roof of our laboratory. Depth-dependent variations of irradiance in the water column were determined using an LI-193SA spherical underwater quantum sensor (LI-COR, USA). The data were normalized with the exponential attenuation phase. The irradiance at a given depth in the water column (Cz) is estimated by Eq. (1) as follows:
where C0, k, and Z are irradiance at the surface, attenuation coefficient, and depth (m), respectively. In the study, the total water column was considered as a euphotic zone to be included in the estimation of primary production because of the shallow depth not over the 3 m even at full tide.Primary production was estimated following Steemann Nielsen (1975) using a hyperbolic function between irradiation and photosynthetic rate, which was saturated from 20.6 × 1015 to 90 × 1015 quanta cm−2 s−1 at 20 °C. The saturation point of the hyperbolic relationship increases with increasing water temperature (Steemann Nielsen 1975). Here, the relationship was modified to a biquadratic model between water temperature and saturated irradiation.
To estimate the primary production, irradiance data must be normalized to 20 °C. If irradiance at depth z (Qz) is less than the saturation point (QR(t)), a direct estimation can be made using the photon flux-photosynthetic rate relationship. If Qz exceeds QR(t), the following two-step normalization is carried out:
where ΔQ is the difference in saturation points between 20 °C and t°C, ΔP is the difference between photosynthetic rates at 20 °C and at t°C, and aP is the linear slope of the photon flux-photosynthetic rate below the saturation point. Therefore, the photosynthetic rate above 20 °C can be obtained using the following equation:To determine the relative photosynthetic rate with respect to chlorophyll-a, PPCz, t is multiplied by chlorophyll-a to calculate the unit time/water volume-dependent primary production (Pz), as follows:
The incident primary production of a water column (PH) can be obtained from the integral of Pz at each depth and euphotic zone, as follows:
where Z and Zd are the depths of the water column. From Eq. (5), the daily primary production can be calculated from the integral of PH during the irradiance period:Results and discussion
Water temperature, salinity, and DO were represented in the range of 8.6–28.0 °C, 26.2–33.6 psu, and 4.9-8.9 mg L−1, respectively, with no significant spatial variation within the five sampling sites (Fig. 2). The maximum water temperatures in the summer off the Wando coast were low compared with those off the Tongyeong coast, which is well known for oyster culturing, and this temperature difference might be attributed to a strong tidal front around Jindo island (Jung 2001; Moon et al. 2006; Oh et al. 2008).
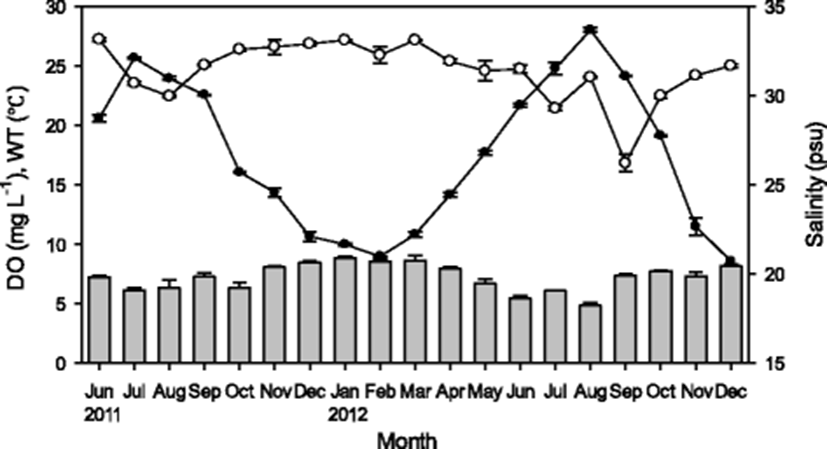
Precipitation can influence the success of oysters (Calvo et al. 1999; Soletchnik et al. 2007), and the input of freshwater can increase PP in coastal waters by the input of land-driven nutrient into the coastal region (Choi et al. 1997). However, no significant regression was observed between salinity and precipitation (P > 0.05) in the present study, which indicates that rainfall is not the main contributor of nutrients to the studied waters. The relatively consistent salinity (30–33 psu) might be influenced by the input of freshwater from adjunct land areas.
The major source of freshwater near the study site is effluent from the tidal gate at Doam-myeon, located ~ 24 km north of the study area. An analysis of tidal residual flow indicates that the effluent from the tidal gate flows mainly out toward Deukryang Bay (Lee and Park 2006), which is located across the channel between Gogeum Island and Maryang-myeon in Gangjin-gun. This flow pattern of the effluent could prevent an abrupt change in salinity after a large rainfall event, which could cause a mass mortality of the rack oyster cultures. The tide with the greatest channel influence on the study area comes from the channel located between Gunwe-myeon in Wando and Pukpyeong-myeon in Haenam-gun, and this waster is weakly mixed with the tide from the Doam effluent between Goma and Sahoo Island.
No clear spatial pattern was observed in the concentration of dissolved inorganic nitrogen (DIN) and dissolved inorganic phosphate (DIP) at the sampling stations located in the seawater surrounding the oyster farm. The measured values were in the range of 0.014-0.062 mg L−1 for NH4-N, 0.001–0.007 mg L−1 for NO2-N, 0.104–0.355 mg L−1 for NO3-N, and 0.011-0.025 mg L−1 for DIP. A seasonal change was observed in nitrate, DIN, and DIP, with a slightly higher concentration in spring and summer than in winter (Fig. 3).
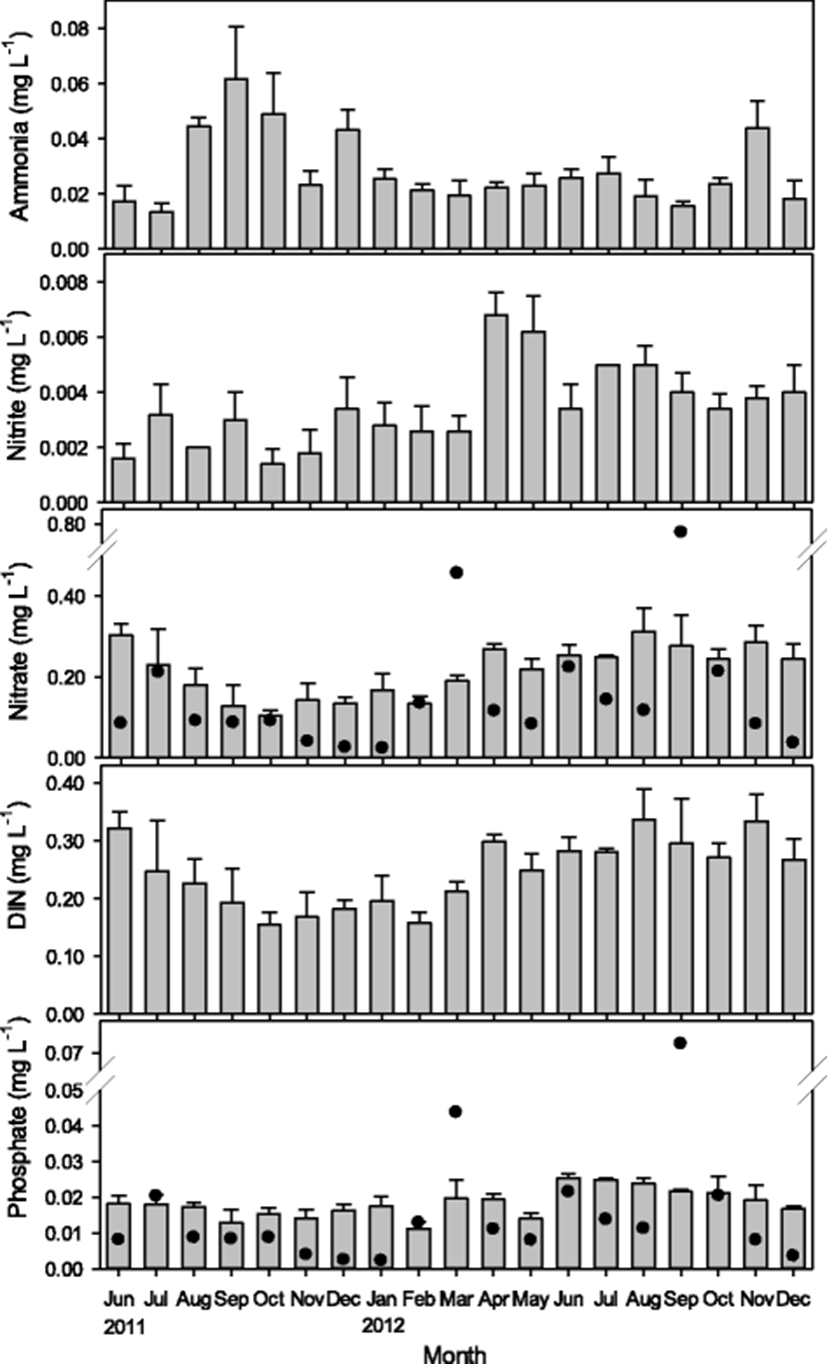
The nitrogen content of the water at surrounding sites was not clearly influenced by precipitation or seasonality (Table 1), which indicated that nitrogen input into this area was weak and relatively consistent. This result could be attributed to the mineralization of resuspended sediment. Resuspension of sediment can double the mineralization rates compared with sediments that are not subjected to turbulence water, regardless of turbulence interval between the events (Ståhlberg et al. 2006). The studied oyster farm is located in the intertidal zone off the Wando coast where the water depth is only 2-3 m at high tide. The sediment could easily be resuspended into the water column by even mild wind and the occasional passing of ships. This resuspension of the sediments might be a source of additional nutrient supply in the waters of the study area. A relatively high tidal current might also contribute to the resuspension of the sediments. The maximum tidal current in the study area is 28.1–29.1 cm s−1 during high tide (Whan et al. 2003; Cho et al. 2013b). This current, combined with increased wave action from various potential sources such as passing ships or wind (Carlin et al. 2016), could be enough to generate sediment resuspension and subsequent mineralization of the sediments.
*P < 0.05; **P < 0.01
Chlorophyll-a content in the study area ranged from 0.31 to 10.46 μg L−1, with an average mean of 2.06 ± 2.10 μg L−1, and was characterized by high temporal variation but no spatial variation (P > 0.05). Peak phytoplankton blooms were observed in the spring and autumn of 2012. The estimated PP ranged from 17.1 to 1052.5 mgC m−2 day−1, with an average of 204.2 ± 224.8 mgC m−2 day−1; however, with the exception of times of intense phytoplankton blooms, the PP was usually below 200 mgC m−2 day−1 (Fig. 4). The trends in PP in this region are similar to those at Gosung Bay on the southern coast and Garorim Bay on the west coast of South Korea (Cho 2013). However, the estimated PP is lower than the values reported for Pukman Bay (Jeong et al. 2009), Gosueng Bay (Lee et al. 2016), Hansan-Geoje Bay (Park et al. 2002), and Kamak Bay (Cho et al. 1996), all oyster farming region off the southern coast of South Korea. The variations in PP in the study area are more similar to those observed off the western coast than the southern coast of South Korea. Primary production is relatively low off the southern coast of South Korea, with reported values below 200 mgC m−2 day−1 throughout the year except during spring and autumn, when phytoplankton blooms generally occur. High turbidity, due to shallow water depth and strong tidal current, could have limited the photosynthetic efficiency of the primary producers in this region (Kuehl and Troelstrup 2013). However, during high tide, the currents could contribute an additional input of chlorophyll-a by freeing benthic diatoms from the sediment (Joint and Pomroy 1981). In the present study, microscopic examination of the phytoplankton composition indicates that benthic diatom species such as Paralia sulcata (Cho et al. 2013b) were relatively scarce. The absence of spring and autumn phytoplankton bloom has been reported to be a distinct feature of waters with high turbidity (Al-Hasan et al. 1975; Joint and Pomroy 1981; Cho 2013); however, the obvious spring and autumn blooms might be attributed to the direct or indirect influence of freshwater input from the adjacent basin and effluent from Doam-myoen at Gangjin-gun.
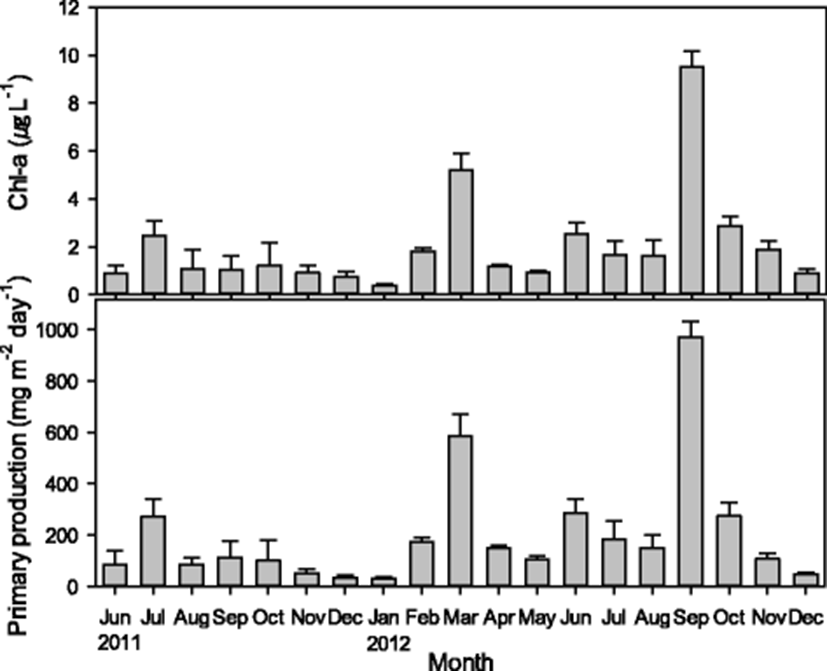
According to the generalized equation for photosynthesis reported by Odum (1971), the availability of nitrate and phosphate for primary production was temporarily limited during the spring and autumn blooming season (Fig. 3). The nutrient availability was limited to a much greater degree in 2012 than in 2011. The hydrodynamic and geological features of this region contribute to the low availability of nutrients in these wasters. The majority of freshwater from the discharge of tidal gate was mostly flowed out toward Deukrayng Bay, meaning that most land-derived input of freshwater in this region consists of runoff, stream water, and hatchery discharge. However, the nutrient load of adjacent basin did not significantly affect the water quality in the study area, as the pollution load showed half dilution within a radius of 300 m (Kang et al. 2015).
Conclusion
Despite the studied waters being located in semi-enclosed ria coast surrounded by several islands, hydrodynamic and geological features make the waters in this area favorable to oyster farming with relatively higher PP. Sediment resuspension might contribute nutrients input that enhance primary production, which satisfied the nutrient demand during the times when phytoplankton blooming are absent; however, during the spring and autumn when phytoplankton blooms occur, this process cannot provide sufficient nutrients for PP due to insufficient nutrient input from the adjacent basin and terrestrial sources.