Background
Melanogenesis is the physiological process that produces melanin pigment, which contributes to skin and hair color (Gilchrest and Eller 1999; Kim et al. 2013). Melanin is the key pigment responsible for skin color in humans. Melanin may be overproduced in melasma, ultraviolet irradiation, and hyperpigmentation diseases. Recently, an increasing number of women desire whiter complexions, especially in Asian countries (Tengamnuay et al. 2006). Therefore, a naturally sourced compound, which not only downregulates melanogenesis but also has no side effects, may be a potential candidate from which to develop a therapeutic agent or cosmetic.
The ocean is an abundant source of both biologically and chemically diverse species. Because of the special environment, marine organisms, including plants, animals, and microorganisms, produce unique metabolites (Kijjoa and Sawangwong 2004; Wang et al. 2016). These metabolites, such as phenolic compounds, carbohydrates, and peptides, possess antioxidant, anti-inflammatory, anti-cancer, anti-obesity, anti-hypertensive, and anti-diabetes bioactivities (Fernando et al. 2017; Kang et al. 2015; Kang et al. 2013; Kim et al. 2016; Kim et al. 2014; Ko et al. 2017; Lange et al. 2015; Lee et al. 2015; Lee et al. 2013; Oh et al. 2016; Samarakoon et al. 2014; Sanjeewa et al. 2016). Ahn et al. (2007) reported on the antioxidant activities of phlorotannins purified from Ecklonia cava, an edible brown alga (Ahn et al. 2007). Ko et al. (2016) purified peptides from flounder and investigated the anti-hypertensive activities of those peptides (Ko et al. 2016). Kim et al. (2014) isolated active compounds from marine bacteria and evaluated their bioactivities (Kim et al. 2014).
Undaria pinnatifida, an edible brown alga, is rich in polysaccharides, especially fucoidan. Park and Choi (2017) isolated different molecular weight fucoidan from U. pinnatifida and investigated their radical scavenging activities and inhibition of melanogenesis (Park and Choi 2017). E. cava is rich in phlorotannins, and we reported their antioxidant and melanogenesis inhibitory activities in a previous study (Ahn et al. 2007; Heo et al. 2009). Glycosaminoglycans (GAGs) are long, unbranched sulfated polysaccharides that possess strong antioxidant activity and possess the potential in cosmetic area (Campo et al. 2004). The objectives of the present study were to evaluate the whitening effects of a fucoidan-rich extract from U. pinnatifida, a phlorotannin-rich extract from E. cava, and GAGs from sea squirt skin. To improve the whitening effects and to reduce the cytotoxicities of these compounds, they were mixed in different ratios and tested to select the mixture that conferred optimal whitening without cytotoxicity.
Materials and methods
Dimethyl sulfoxide (DMSO), 3-(4-5-dimethyl-2yl)-2-5-diphynyltetrasolium bromide (MTT), mushroom tyrosinase, and alpha-melanocyte-stimulating hormone (α-MSH) were purchased from Sigma Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle medium (DMEM), penicillin/streptomycin, and fetal bovine serum (FBS) were purchased from Gibco BRL (Life Technologies, Burlington, ON, Canada). Antibodies against tyrosinase, tyrosinase-related protein-1 and protein-2 (TRP-1 and TRP-2), and microphthalmia-associated transcription factor (MITF) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-mouse and anti-rabbit IgG were purchased from Cell Signaling Technology (Beverly, MA, USA). All other chemicals were of analytical grade.
U. pinnatifida was hydrolyzed with Celluclast. The polysaccharide fraction was precipitated with ethanol and referred to as UPEF. UPEF contains 36.10 ± 3.20% fucoidan. An 80% ethanol extract of E. cava was prepared (ECE), and it contained 26.85 ± 0.16% phenols. The sea squirt skin was hydrolyzed with Celluclast, and glycosaminoglycans (GAGs) were separated and purified; the purity of GAGs was 95%. All samples were stored at − 20 °C until use. The sample mixtures were prepared by mixing each component solution at the described ratios.
The inhibition of mushroom tyrosinase was measured as previously described (Heo et al. 2009; Kang et al. 2012). In brief, the 200-μL assay mixture in a 96-well microplate contained 40 μL of 1.5-mM L-tyrosine, 140 μL of 50-mM phosphate buffer (pH 6.5), 10 μL of aqueous mushroom tyrosinase (1000 units/mL), and 10 μL of test solution. The assay mixture was incubated at 37 °C for 12 min and then kept on ice for 5 min to stop the reaction. The amount of dopachrome in the reaction mixture was measured at 490 nm using a microplate reader (BioTek Synergy HT, BioTek Instruments, Winooski, VT, USA).
B16F10 mouse melanoma cells (ATCC® CRL-6475™) were purchased from ATCC (American Type Culture Collection, Manassas, VA, USA) and grown in DMEM supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were incubated in an atmosphere of 5% CO2 at 37 °C and were sub-cultured every 4 days. Cells for experiments were seeded at a concentration of 5 × 104 cells/mL.
Cell viability was quantified by a colorimetric MTT assay (Wang et al. 2017; Wang et al. 2018). Briefly, B16F10 cells were seeded in a 96-well plate and incubated for 24 h. The cells were treated with different concentrations of test samples and incubated for 72 h. MTT solution (50 μL, 2 mg/mL) was added to each well and incubated for 3 h. The supernatant was aspirated, 150 μL of DMSO was added to each well, and the absorbance was measured at 540 nm using a microplate reader.
B16F10 cells were seeded in a 6-well plate and incubated for 24 h. The cells were treated with various concentrations of test samples and stimulated with α-MSH (50 nM). After 72 h, the cells were washed with ice-cold PBS and harvested. The harvested cells were incubated at 80 °C for 1 h in 1 mL of 1-N NaOH containing 10% DMSO. The absorbance of the supernatant was measured at 490 nm using a microplate reader (Heo et al. 2010).
The effect of the test samples on the expression of melanogenesis-related proteins including MITF, tyrosinase, TRP-1, and TRP-2 was assessed by Western blot analysis as described previously (Kim et al. 2013). In brief, B16F10 cells were incubated with various concentrations of the test sample and stimulated with α-MSH (50 nM). After 72 h, cells were harvested and lysed. The protein content of each sample was measured with a BCA™ kit. The proteins (50 μg) were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were incubated in blocking buffer (5% skim milk) and then with primary antibodies for 16 h at 4 °C. The membranes were then incubated with secondary antibody at room temperature for 3 h. Finally, the proteins were visualized using an ECL Western blotting detection kit and exposure to X-ray film.
All experiments were performed in triplicate. The data were expressed as means ± standard errors (S.E). The mean values of each experiment were compared using one-way ANOVA. Significant differences between the means were determined by the Duncan test. A p value < 0.05 was considered to be statistically significant, and degrees of significance were indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ###p < 0.001.
Results
The effects of UPEF, ECE, and GAGs on tyrosinase activity were examined by measuring L-tyrosine hydroxylation. Arbutin was used as a positive control. As Fig. 1 shows, UPEF, ECE, and GAGs inhibited tyrosinase activity in a dose-dependent manner. ECE showed the strongest inhibition of the three agents, and it inhibited tyrosinase activity by 64.33% at a concentration of 100 μg/mL.
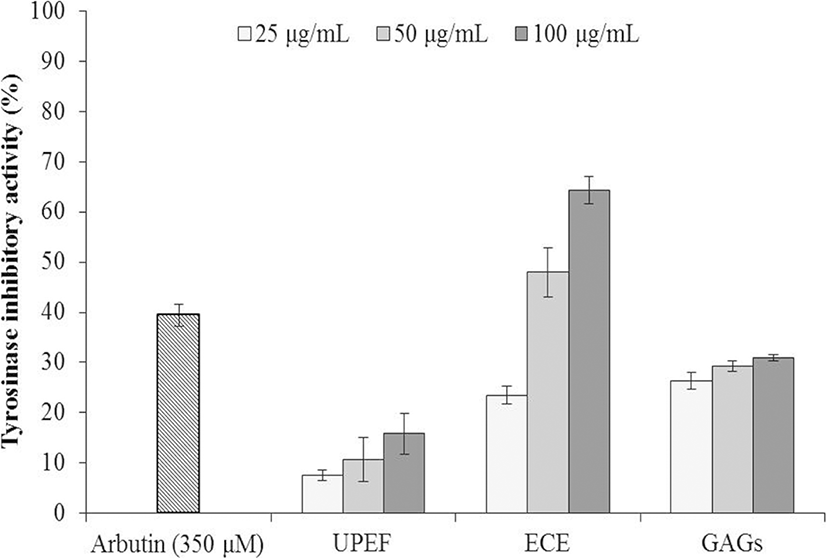
The cytotoxicities of UPEF, ECE, and GAGs on B16F10 cells were examined by MTT assay, and the results are summarized in Fig. 2a. As the results show, ECE caused significant cytotoxicity in B16F10 cells. In addition, UPEF and GAGs were slightly toxic to B16F10 cells at high concentration (100 μg/mL).
As Fig. 2b indicates, the melanin content of cells not stimulated with α-MSH is referred to as 100%, and the melanin content of cells stimulated with α-MSH increased by 80%. The melanin content of cells treated with UPEF, ECE, and GAGs decreased in a dose-dependent manner. These results indicated that all three test substances inhibited melanogenesis in α-MSH-stimulated B16F10 cells, and ECE showed the strongest effect.
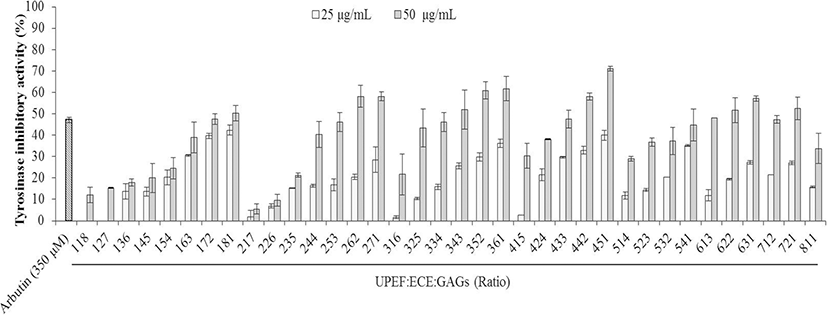
UPEF, ECE, and GAGs were combined in various ratios by volume, and the effect on tyrosinase activity was measured. As Fig. 3 shows, tyrosinase activity decreased as the ECE ratio in the mixture increased. In addition, the inhibitory activities of the test agents in combination were stronger compared to each agent alone at the same concentration.
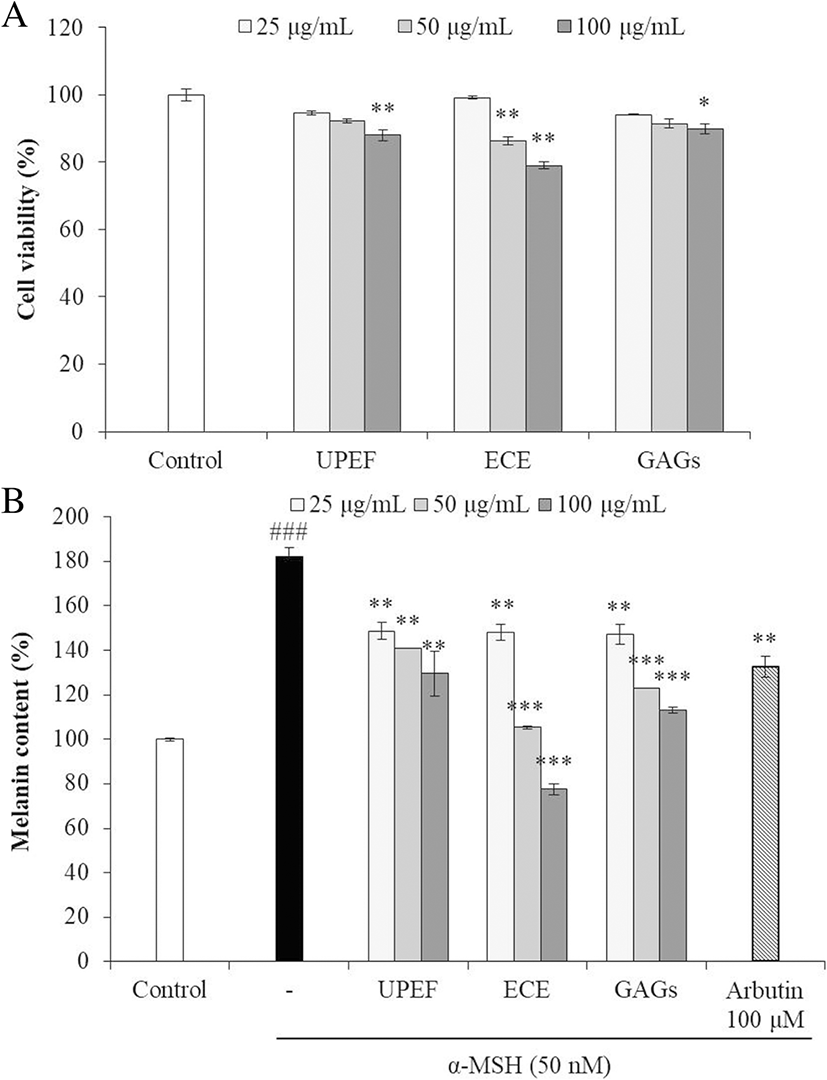
Using the tyrosinase inhibition conferred by the test agents in combination, seven combinations (UEG-262, UEG-271, UEG-352, UEG-361, UEG-433, UEG-451, and UEG-721) were selected to test the effects on melanogenesis in B16F10 cells. The cytotoxicities of these combinations in B16F10 cells were measured by MTT assay. As Fig. 4a shows, most combinations were slightly toxic to B16F10 cells, and UPEF to ECE to GAGs at 2:6:2 (UEG-262) showed the strongest cytotoxicity. On the other hand, UEG-451 showed no cytotoxicity in B16F10 cells.
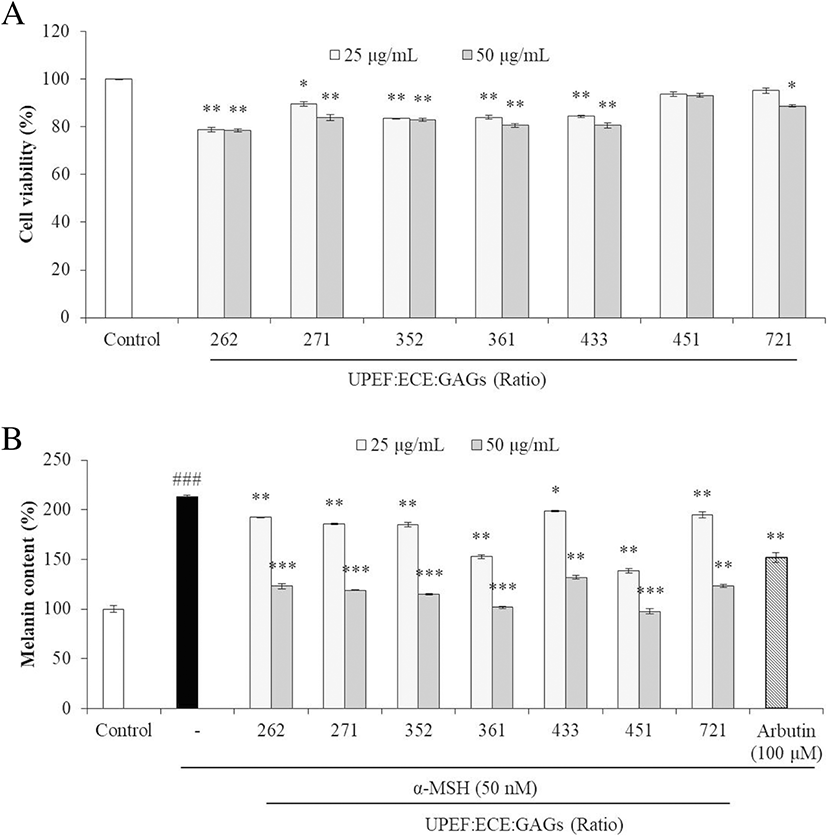
The effects of the test agents in combination on melanogenesis were assessed by measuring melanin synthesis in α-MSH-stimulated B16F10 cells. As the results show (Fig. 4b), all combinations significantly reduced melanin synthesis in α-MSH-stimulated B16F10 cells, especially UEG-451. These results show that UPEF, ECE, and GAGs in combination inhibited melanogenesis, and 4:5:1 is the optimum ratio.
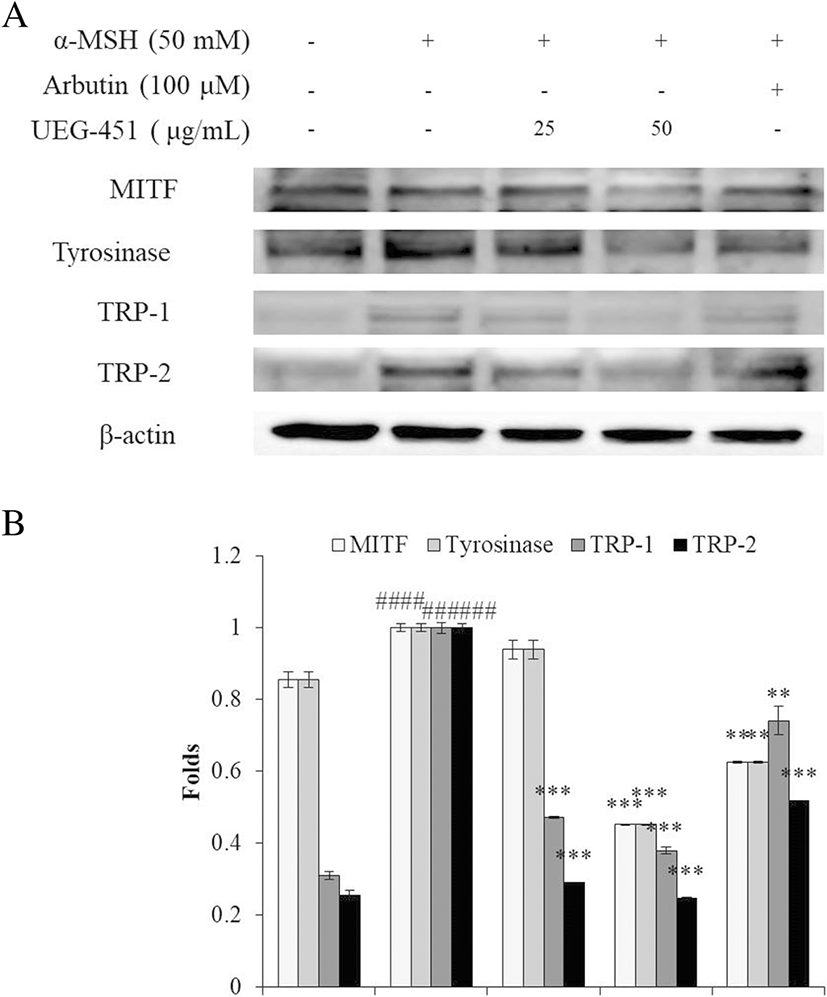
Based on its cytotoxicity and inhibition of melanogenesis, UEG-451 was selected for further research to evaluate the mechanisms of its anti-tyrosinase and anti-melanogenesis activities. The effect of UEG-451 on the expression of tyrosinase, TRP-1, TRP-2, and MITF was examined by Western blot analysis. As shown in Fig. 5, the expressions of tyrosinase, TRP-1, TRP-2, and MITF were increased by α-MSH stimulation and the expressions of these proteins were reduced in cells pre-treated with different concentrations of UEG-451.
Discussion
In Asian cultures, lighter skin tones are considered more desirable. To meet the needs of the many women who suffer from hyperpigmentation, whitening agents have been developed for cosmetic and medical applications. Various whitening cosmetics or cosmeceuticals, which are made from natural or chemosynthetic materials, are commercially available. However, some of these materials possess side effects or are toxic because they contain substances such as hydroquinone and heavy metals. Thus, more research is devoted to searching for safe and effective whitening agents from natural sources.
Many studies have reported the effects of plant extracts or compounds isolated from plants on melanogenesis (Arung et al. 2011; Chan et al. 2011). Arung et al. (2011) isolated quercetin and its derivatives from Allium cepa and investigated their anti-melanogenesis effects (Arung et al. 2011). Chan et al. (2011) evaluated the tyrosinase inhibition and melanin synthesis inhibition in B16F10 cells of Sargassum polycystum ethanolic extracts and their fractions (Chan et al. 2011). In the present study, we evaluated melanogenesis inhibition conferred by a fucoidan-rich extract, a phlorotannin-rich extract, and GAGs obtained from seaweed and sea squirt, and investigated their whitening effects when in combination.
The effects of UPEF, ECE, and GAGs on commercial mushroom tyrosinase were investigated, and the results indicated that all samples possess tyrosinase inhibitory activity with ECE showing the strongest activity of the three (Fig. 1). In addition, UPEF, ECE, and GAGs significantly reduced α-MSH-induced melanin synthesis in B16F10 cells in a dose-dependent manner (Fig. 2). ECE showed stronger melanin synthesis inhibition activity than UPEF and GAGs, and the melanin content of cells treated with 100 μg/mL of ECE was lower than the unstimulated cells. These results demonstrated that ECE is a potent inhibitor of melanin synthesis in α-MSH-stimulated and non-stimulated B16F10 cells. However, all three samples were cytotoxic to B16F10 cells, especially ECE. Therefore, we mixed the test agents in different ratios and compared the whitening effects and toxicities of the combinations to those of the agents alone.
The inhibition of tyrosinase and melanin synthesis conferred by the combinations was stronger than each single agent at the same concentrations (Fig. 1 and Fig. 3; Fig. 2b and Fig. 4b); UEG-451 showed the strongest activity and no toxicity, with 71.10% of control tyrosinase activity and a 115.24% reduction in α-MSH-stimulated melanin synthesis at 50 μg/mL. Combining the agents at an optimal ratio may be an ideal and effective way to improve bioactivity and reduce cytotoxicity.
Melanogenesis is regulated by enzymes including tyrosinase, TRP-1, and TRP-2. Tyrosinase is considered to be the rate-limiting enzyme of melanin biosynthesis and represents the major regulatory step in melanogenesis (Maeda et al. 1997). Thus, the inhibition of related enzymes is the most common approach to developing skin-whitening agents. The present results revealed that UEG-451 inhibited tyrosinase and reduced melanin synthesis in α-MSH-stimulated B16F10 cells. In addition, Western blot results demonstrated that the expressions of tyrosinase, TRP-1, and TRP-2 in UEG-451-treated cells decreased in a dose-dependent manner compared to non-treated cells (Fig. 5a and b). The tyrosinase family genes, TRP-1 and TRP-2, which are responsible for melanin synthesis, are regulated by MITF (Levy et al. 2006). As Fig. 5 shows, UEG-451 at 50 μg/mL significantly reduced MITF expression in α-MSH-stimulated B16F10 cells. These results suggest that UEG-451 inhibition of α-MSH-induced melanogenesis in B16F10 cells may be through the downregulation of tyrosinase, TRP-1, and TRP-2 via inhibition of MITF expression.
Conclusions
The present study investigated the inhibition of melanogenesis conferred by natural marine bio-resources including UPEF, ECE, and GAGs in α-MSH-stimulated B16F10 melanoma cells. The results indicated that all three agents inhibited tyrosinase and melanin synthesis in α-MSH-stimulated B16F10 cells and that they can act synergistically when in combination. In addition, UPEF, ECE, and GAGs in the ratio of 4:5:1 (UEG-451) showed the strongest activity and was not toxic. These results suggest UEG-451 may be an ideal whitening agent for use in the medical and cosmetic industries.