Background
The ocean is a large body of water that covers over 70% of earth embedding numerous resources including potent therapeutic agents (Fernando et al. 2016). Ocean current is non-predicted directional movement of both warm and cold current, which provides the best ground for marine organisms. Accordingly, the East Sea of South Korea including Dokdo island is affected by North Korean cold current and Tsushima warm current (Ryu et al. 2012; Yun et al. 2004). Due to this parallel current, high pressure and high salinity due to sea depth over 400 m, and its geological positioning, the East Sea is known as the Pacific Ocean with nutrient-rich environment (Rho et al. 2016; Danovaro et al. 2017). Moreover, the East Sea is a unique habitat for unique organisms due to the characteristic features of the deep sea such as low intensity of light which may be not enough to support photosynthesis, low oxygen concentration, and declining temperature with increasing depth (Yoon and Chough 1995). Thus, the marine organisms inhabited in the deep sea have adapted to extreme conditions, which causes the development of unique and special metabolites including tremendous polyphenolic compound and polysaccharides (Gomes et al. 2016).
Marine organisms have been reported to possess nutraceutical and pharmaceutical potentials in human health (Chandika et al. 2015; Najafian and Babji 2017). Especially, marine algae are a great source of polyphenolic compounds like phlorotannins with various bioactivities (Wijesinghe and Jeon 2011). Also, marine invertebrates such as sponges, soft corals, starfish, and sea squirt produce various secondary metabolites in their defense system against predators and microorganism infection, which has a potential biological effect on human health (Ko et al. 2017). However, few studies have been reported on the secondary metabolites of marine invertebrates from the East Sea of South Korea (Ko et al. 2017).
Thus, the aim of this study is to investigate the chemical compositions and biological activities of marine invertebrates such as Crossaster papposus japonicus, Actinostola carlgreni, Stomphia coccinea, Actinostola sp., and Heliometra glacialis collected from the East Sea of South Korea.
Materials and methods
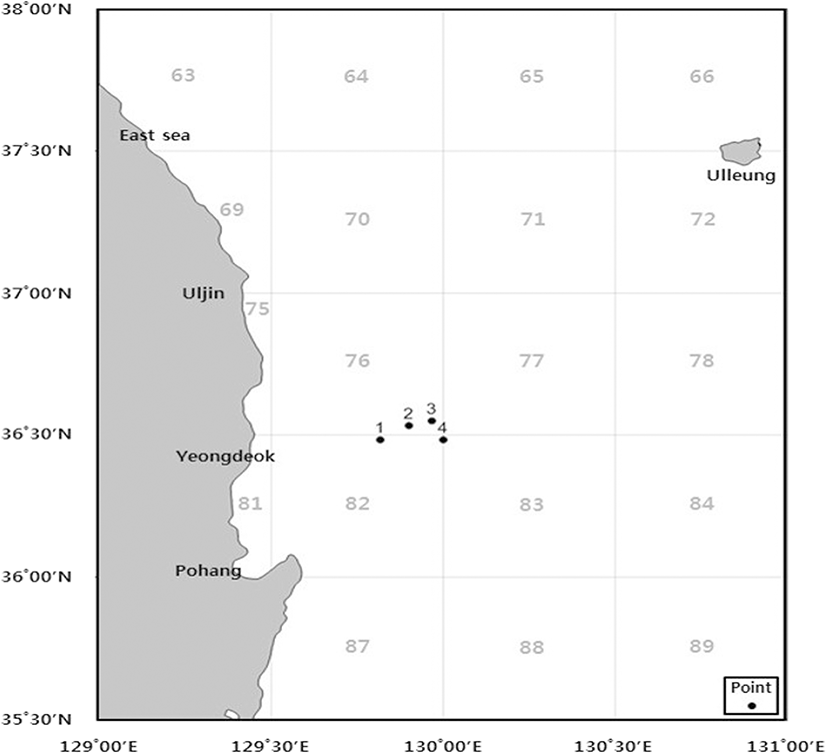
Five species of marine invertebrates (C. papposus japonicus, A. carlgreni, S. coccinea, Actinostola sp., and H. glacialis) were collected from the deep ocean seafloor (depth range 300–1000 m) around Wangdol-cho in the southwest area of East Sea during trawl survey of National Institute of Fisheries Science in June 2017 (Fig. 1). The marine invertebrates were washed three times with tap water to remove salt, sand, and epiphytes attached to their surface and then rinsed with distilled water and freeze at − 80 °C. The frozen samples were lyophilized and homogenized using home grinder prior to extraction.
Lipopolysaccharide (LPS), Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride in 2.5% phosphoric acid), and 3-(4, 5–dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich, St. Louis, USA. Dulbecco’s minimum Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from GIBCO™, Invitrogen Corporation, Carlsbad, CA, USA. The other chemical and reagents were used of analytical grade, EtOH.
Freeze-dried marine invertebrates were mixed with 70% EtOH at a ratio of 1:10 (w/w) and then subjected to continuous shaking at room temperature for 24 h. The liquid layer was centrifuged for 20 min to remove the residue and filtered under reduced pressure. The filtrates were volatilized with a vacuum concentrator under reduced pressure, and the concentrates were freeze-dried to obtain extracts.
Freeze-dried marine invertebrates were mixed with distilled water at a ratio of 1:10 (w/w) and then subjected to continuous shaking in a water bath at 90 °C for 3 h. The extracted solution was centrifuged for 20 min to remove the residue and filtered under reduced pressure. The filtrates were freeze-dried to obtain dry powder of extracted samples. The extracts were kept at − 70 °C for further use.
Protein contents were determined using bicinchoninic acid (BCA) protein assay kit (Thermo, Rockford, IL, USA) following the manufacturer's specification. Briefly, 20 μl of each extract with 180 μl of working reagent solution was incubated at 37 °C for 30 min. Absorbance was measured at 562 nm using a microplate reader (PowerWave XS2, BioTek Instruments, Inc., Winooski, VT, USA). A bovine serum albumin standard curve was prepared to calculate the protein content.
Polyphenol contents were measured according to a protocol previously described by Singleton et al. (1999). Two hundred fifty microliters of 7.5% Na2CO3 was added to 100 μl of each extract and reacted at room temperature for 5 min. Then, 300 μl of 1N Folin-Ciocalteu reagent was added and incubated in a dark condition for 30 min. After incubation, absorbance was measured at 765 nm using a microplate reader. A gallic acid standard curve was prepared to calculate the polyphenolic content.
Sugar contents were measured according to a protocol previously described by Dubois et al. (1956). One hundred microliters of each extract was mixed with 100 μl of 5% phenol and 500 μl of H2SO4 and reacted at room temperature for 20 min. The absorbance was measured at 490 nm using a microplate reader. A glucose standard curve was prepared to calculate the sugar content.
Antioxidant activity was determined by ABTS radical scavenging assay according to the method used by Thaipong et al. (2006). The ABTS radical was generated by 2.45 mM potassium persulfate and 7 mM 2,2′-azino-bis (ehtylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) reaction for 16 h at room temperature. Then, a mixture was diluted with distilled water and obtained the absorbance at 735 nm using a microplate reader. Then, 100 μl of each extract was mixed with 100 μl of ABTS radical solution at room temperature for 30 min in a 96-well plate and the absorbance of mixture was measured again at 735 nm using a microplate reader. Finally, the IC50 value, the concentration required for 50% scavenging of ABTS radical, was determined.
The ABTS radical scavenging activity was calculated as follows:
ABTS radical scavenging activity (%) = [1 − (Ac − As)/Ac] × 100
where Ac is the absorbance of the control sample and As is the absorbance of the sample solution.
The murine macrophage cell line RAW 264.7 was purchased from the American Type of Culture Collection (Rockville, MD, USA). RAW 264.7 cells were cultured in DMEM supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% FBS. The cells were then incubated in an atmosphere of 5% CO2 at 37 °C and subcultured every 2 days.
Cytotoxicity assessment was performed by MTT assay. RAW 264.7 macrophages (4 × 105 cells/ml) plated on 24-well plates were preincubated at 37 °C for 24 h. Cells were treated with extracted samples at various concentrations (100, 200, and 400 μg/ml) and incubated under the same conditions. After 1 h of incubation, LPS (0.25 μg/ml) was added to the cell culture medium and incubated again at 37 °C for 24 h. MTT stock solution (100 μl; 1 mg/ml) was added to each well and further incubated for 4 h allowing formazan formation in the viable cells. Thereafter, supernatants were removed. The formazan crystals in each well were dissolved in 100 μl of dimethyl sulfoxide (DMSO). The absorbance was measured at 540 nm using a microplate reader.
After 24 h preincubation of RAW 264.7 macrophages (4 × 105 cells/ml) at 24-well plates with various concentrations (100, 200, and 400 μg/ml) of extracts and with LPS (0.25 μg/ml), the quantity of nitrite accumulated in the culture medium was determined as an indicator of NO production. NO production was measured using 100 μl of cell culture medium mixed with 100 μl of Griess reagent. The mixture was then incubated for 10 min, and the absorbance was determined at 540 nm in a microplate reader.
Type bacterial strains used in the present study were obtained from the Korean Collection for Type Cultures (KCTC; Daejeon, Korea): Staphylococcus aureus (KCTC 1916) and Escherichia coli (KCTC 2593). S. aureus was grown aerobically at 37 °C.
The antibacterial activity of extracts was determined by disc diffusion assay. A suspension of each bacteria was spread on the Mueller-Hinton agar (MHA) plates, and paper discs (6 mm in diameter) containing 1 and 5 mg of each extract was placed on the surface of inoculated MHA plates. After incubation at 37 °C for 24 h, the diameter of growth inhibition zone was measured using vernier calipers.
The minimum inhibitory concentration (MIC) assay was followed by the guideline of Clinical and Laboratory Standards Institute (2015). The MIC assay was performed using 2-fold dilution method with Mueller-Hinton broth (MHB) in 96-well microplates. The MIC values were determined visually.
All data were expressed as mean ± standard deviation (SD) of three replications. Statistical analysis was performed using one-way ANOVA, followed by Duncan’s multiple range test using the SPSS program (SPSS Inc. Ver12.0). The differences were considered statistically significant at p < 0.05.
Results and discussions
Table 1 shows the yields of marine invertebrates extracted using 70% EtOH and hot water in a percent of freeze-dried weight of marine invertebrates. The yields of 70% EtOH extracts showed a wide deviation in yield ranging from 5.18 ± 0.52 to 31.20 ± 0.11% (w/w) compared to hot water extraction 21.50 ± 1.40 to 37.70 ± 1.38% (w/w). In addition, hot water extracts gave a moderately higher percentage of yields compared with 70% EtOH, except A. carlgreni, which gave a relatively low yield (28.20 ± 2.35% w/w). These results demonstrate that the hot water extraction is the most efficient method for obtaining higher yields compared to 70% EtOH extraction.
The marine invertebrates are commonly composed of a greater amount of water, protein, and minerals and a few amounts of sugars and phenolic substances. For example, the red sea anemone (Actinia equine), one of the most common sea anemones, is also composed of 80% water, 13% protein, and some of sugars and minerals (Silva et al. 2017). Furthermore, the abalone contains about 70% water and 20% protein (Qian et al. 2012). The chemical compositions of marine invertebrate’s extracts in our present study are presented in Table 2, which shows a higher percentage of protein, both in 70% EtOH and hot water extracts as a common feature of the marine invertebrates. Similar to that of the total yield of the crude extraction, the protein content of the 70% EtOH extracts exhibited the higher deviation while hot water extraction exhibited the minor deviation and higher protein content compared to 70% EtOH extracts. However, some hot water extracts showed lower polyphenol content than in 70% EtOH extracts, where only A. carlgreni showed lower sugar content in hot water extraction. Moreover, both polyphenol and sugar contents in both hot water and 70% EtOH extracts showed a significantly lower amount than the protein content, which illustrates the same common feature of marine invertebrates.
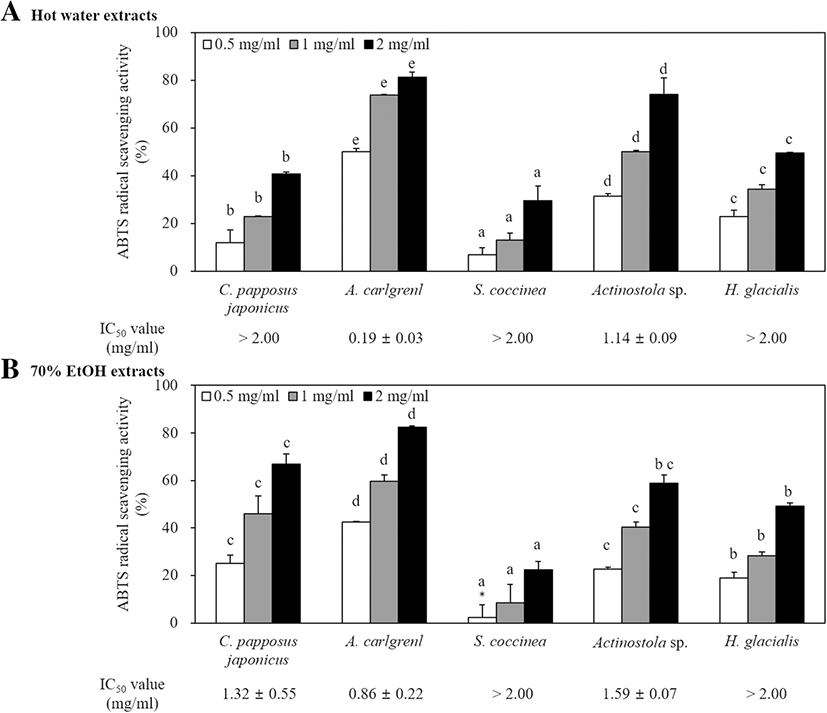
In this study, antioxidant activities were tested and compared using ABTS radical scavenging activity. The antioxidant activities of extracts are shown in Fig. 2. Among them, 70% EtOH extract of A. carlgreni showed the highest scavenging activity as 42.4, 59.64, and 82.5% at the concentrations of 0.5, 1, and 2 mg/ml, respectively. The IC50 value of A. carlgreni exhibited the lowest both in hot water and 70% EtOH extract, and the values were 0.19 ± 0.03 mg/ml and 0.86 ± 0.22 mg/ml, respectively. Further similar studies, the antioxidant activities of Acanthaster planci (Lee et al. 2014a), Ophiocoma erinaceus (Amini et al. 2015), and Edwardisa sipuncluoides (Rongjun et al. 2015) reported higher ABTS radical scavenging activities. However, 70% EtOH and hot water extracts obtained from A. carlgreni exhibited significantly higher antioxidant activities with respect to A. planci (IC50 value, 1.62 mg/ml) and O. erinaceus (IC50 value, 1.012 mg/ml) activities. Moreover, similar antioxidant activity was reported by E. sipuncluoides (IC50 value, 0.25 mg/ml) to hot water extract of A. carlgreni.
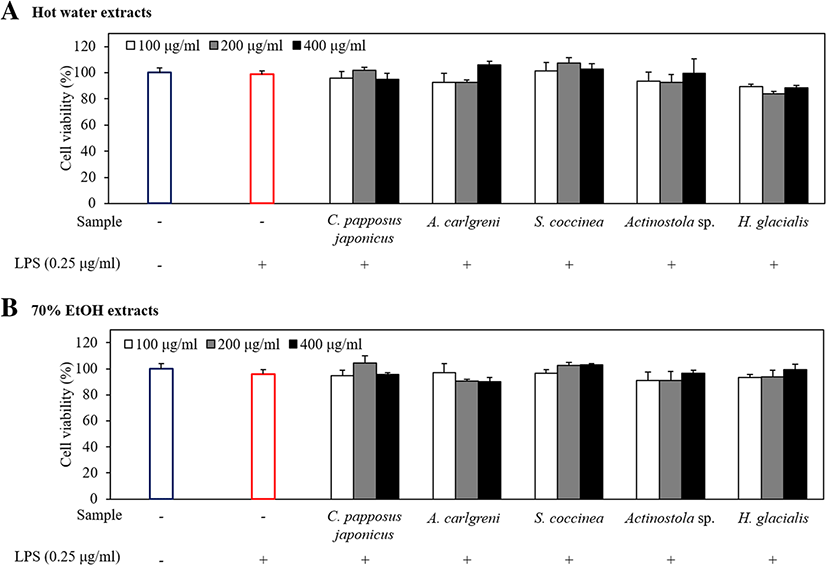
A tiny amount of NO (10− 12 mol) secreted under normal conditions of cells plays a variety of physiological roles, including neurotransmission, relaxation of vascular smooth muscle, and inhibition of platelet aggregation (Sanders and Word 1992). However, a higher concentration of NO (10−19 mol) secreted under abnormal conditions produces strong hydroxyl radical and harmful substances and causes the deamination of intracellular DNA which leads to cell damages and apoptosis (Beckman et al. 1990). Prior to evaluating the inhibitory effect of marine invertebrate extracts on NO production, we first examined their cytotoxicity in LPS-stimulated RAW 264.7 macrophages using MTT assay. According to the results, all extracts did not exhibit cytotoxicity at different concentrations: 100, 200, and 400 μg/ml (Fig. 3). To evaluate the anti-inflammatory activity of marine invertebrate extracts on NO production, RAW 264.7 macrophages were stimulated with LPS in the absence or presence of all extracts. The LPS-stimulated group distinctly induced NO production compared with the non-stimulated group (Fig. 4).
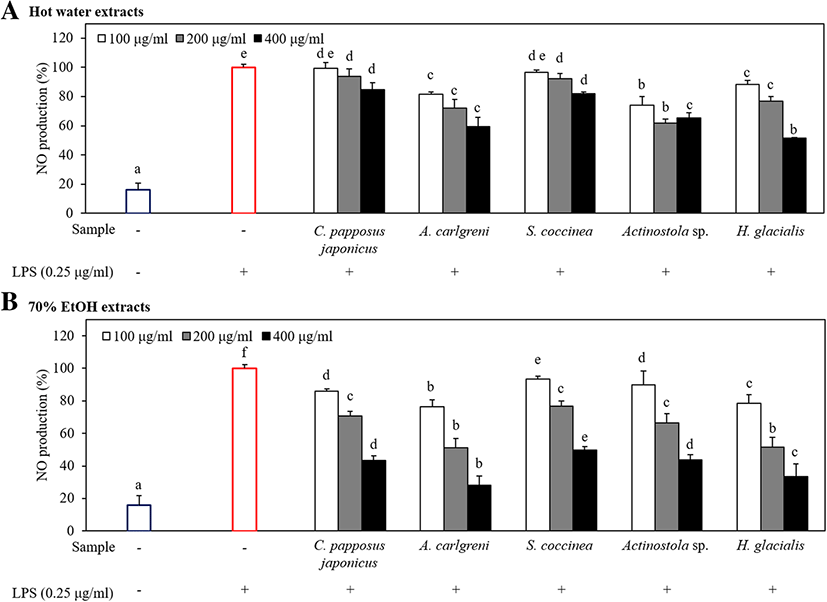
As shown in Fig. 4, all 70% EtOH extracts inhibited the NO production compared with the respective hot water extracts in all level of concentrations. However, both types of extracts of A. carlgreni showed higher anti-inflammatory activities than other extracts while 70% EtOH extract of A. carlgreni indicates 71.8% reduction of NO production in LPS-stimulated RAW 264.7 macrophages at the concentration of 400 μg/ml. In addition, the hot water extract of H. glacialis showed the highest anti-inflammatory activity at the concentration of 400 μg/ml indicating 48.5% inhibition in NO production.
Similar to the present study, Senthilkumar and Kim 2013 investigated the anti-inflammatory activity of few marine invertebrate-derived compounds, since marine invertebrates possess vital bioactive compounds and they have found excellent anti-inflammatory action on human neutrophils by “ascidiathizone” isolated from Ascidian Aplidium. Moreover, “Cembranolides” isolated from Lobophytum crassum also showed the higher potential as an anti-inflammatory drug through inhibiting COX-2. In addition, Senthilkumar and his team further showed that “Plakortide P” is isolated from P. angulospiculatus with outstanding anti-neuroinflammatory activity (Senthilkumar and Kim 2013).
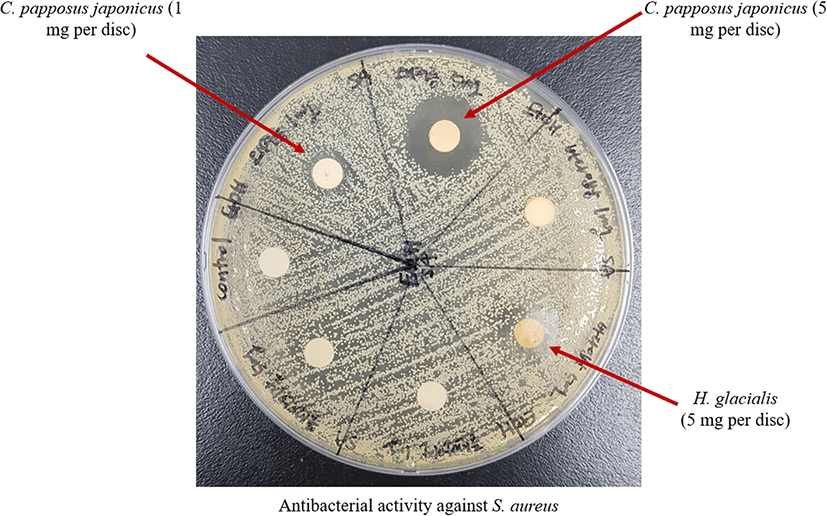
The antibacteril assay were carried out by disc diffusion method against two bacterial species (E. coli and S. aureus) (Lee et al 2014b). Table 3 shows the antibacterial activities of the extracts. Among them, the diameter of clear zone of 70% EtOH extracts of C. papposus japonicus and H. glacialis against S. aureus was 15.47 and 11.78 mm, respectively. In addition, Fig. 5 illustrates the antibacterial activity of C. papposus japonicus and H. glacialis 70% EtOH extracts against S. aureus and the antibacterial activity of each sample was demonstrated through a zone of inhibition. Moreover, C. papposus japonicus 70% EtOH extract gave a clearer zone of inhibition demonstrating a significant antibacterial activity against S. aureus compared to the others.
Moreover, the antibacterial activity against E. coli and S. aureus were evaluated by MIC assay as resulted in Table 4. Among the 70% EtOH extracts, the C. papposus japonicus extract showed the lowest MIC value (256 μg/ml) against S. aureus demonstrating the complete inhibition of S. aureus at lower sample concentration. The MIC value of H. glacialis extract against S. aureus was determined at a concentration of 512 μg/ml. However, the antibacterial effects on E. coli did not mark values (> 1024 μg/ml) which indicate all extracts were less effective on E. coli. Hence, the results suggested that both 70% EtOH extract of C. papposus japonicus and H. glacialis significantly present antibacterial substance against S. aureus.
Many investigators including Natarajan and his colleagues have found the outstanding antibacterial activity of crude extracts of marine invertebrate (Daletos et al. 2016; Natarajan et al. 2010). They have found that methanol extract of Polyclinum madrasensis could inhibit the activity of S. aureus through 23 mm of zone of inhibition at a concentration of 4 mg/ml (Natarajan et al. 2010), which was a greater inhibitory effect compared with the 70% EtOH extract of C. papposus japonicus and H. glacialis. However, the methanol extraction of P. madrasensis exhibited a significantly higher MIC value (700 μg/ml) (Natarajan et al. 2010) compared with the 70% EtOH extract of C. papposus japonicus against S. aureus. Hence, these studies reveal that 70% EtOH extract of C. papposus japonicus is expected to have potential therapeutic agents to treat skin infections (Oh et al. 2017).
Conclusions
This study was focused on chemical compositions and biological activities of marine invertebrates such as Crossaster papposus japonicus, Actinostola carlgreni, Stomphia coccinea, Actinostola sp., and Heliometra glacialis collected from the East Sea. Thus, we conducted ABTS radical scavenging assay to measure antioxidant activities and inhibition of NO production to measure the anti-inflammatory activity on LPS-stimulated RAW 264.7 macrophages. The 70% EtOH extract of A. carlgreni demonstrated the highest free radical scavenging activity (IC50 value 0.19 ± 0.03 mg/ml) and anti-inflammatory activity among all extracts obtained from marine invertebrates. MTT assay indicated that all extracts treated under 400 μg/ml of concentration have no cytotoxicity on the macrophages. Moreover, the 70% EtOH extract of C. papposus japonicus showed the widest clear zone of inhibition (15.47 mm) and lowest MIC value (256 μg/ml) against S. aureus indicating the highest antibacterial activity. Overall, the results suggest that marine invertebrate-derived compounds are a tremendous pharmaceutical agent in prospect drug development over synthetic drugs.