Background
Intensification of aquaculture makes fishes more prone to outbreak of many infectious diseases. High intensity of fishes and shortfall of hygienic conditions not only diminish the fishes well-being but also increase the probability of fish diseases due to the facilitation of pathogen dispersal (Quesada, Paschoal, and Reyes, 2013). As fish demand is continuously increasing with the increasing human population, there is an immediate need for increased disease resistance, growth performance, and feed efficiency in intensified fish culture without the usage of antibiotics or chemotherapeutics. Conventionally used antibiotics, chemotherapeutics for improving fish health (Ghosh, Sinha, and Sahu, 2007), many antimicrobials, and other veterinary drugs that may act as prophylactics and growth performers (Rico et al., 2013) are not safe for the environment as well as for human health, and these drugs may result in the development of a resistant strain of bacteria and virus (Seyfried, Newton, Rubert, Pedersen, and McMahon, 2010). Synthetic immunostimulants could have a good approach towards replacing these antimicrobials and chemotherapeutics but to a limited extent due to its retaining residues in the environment. So there is a drift towards the use of herbal and plant extracts to manage disease in aquaculture as these are environment-friendly methods that have the potential to improve aquatic prosperity. Among plants, Mentha piperita (peppermint) is a hybrid mint produced by the cross of water mint and spearmint and also one of the most important medicinal plants produced all over the world. Mentha sp. have some significant properties like anticancer and anti-inflammatory (Blumenthal, 1998) and many remarkable impacts on hematological and immunological responses (Nobakht and Mehmannavaz, 2010). Furthermore, many reported literature have proven that peppermint acts as an important feed additive for increasing growth performance and immune response as well as disease resistance for different fishes.
Probiotics have also gained appreciation as an approach towards combating and controlling diseases in aquaculture. According to FAO/WHO (2001), probiotics are live microbes which when administered in suitable extent confers health assistance on the host. Several species of different bacteria such as Bacillus in Catla catla and Cirrhinus mrigala (Bandyopadhyay, 2004), Lactobacillus (Balcazar et al., 2007), and Aeromonas and Pseudomonas (Nayak and Mukherjee, 2011) in different carp species have been isolated and purified from the fish gut and integrated in formulated diet to study their effects on growth and immunity. In earlier studies, in our laboratory, attempts have been made to use commercial probiotics in fish feed (Sushma, 2007). Studies were further undertaken and Bacillus coagulans CCI was isolated as autochthonous probiotic bacteria from the fish gut of Catla catla (Bhatnagar, Raparia, and Kumari, 2012) and evaluated that the effect of dietary incorporation of isolated Bacillus coagulans CC1 resulted in enhanced growth performance and nutrient retention when incorporated in fish diet at an optimum level (Bhatnagar and Raparia, 2014). C. catla is one of the important Indian Major Carps and a very constitutive part of our food chain. Its economic importance is gradually declining due to its disease outbreak. Plant extracts along with probiotics can strongly make an alternative way to replace synthetic immune stimulants and enhance the aquatic health leading to sustainable aquaculture. However, to the best of author’s knowledge, literature is not available demonstrating the use of plant extract and autochthonous probiotics in synergism. Therefore, the current study was undertaken to assess the impact of M. piperita and B. coagulans on growth performance, nutritional physiology, and immunity of C. Catla.
Materials and methods
The experimental fish fingerlings of Catla catla were procured from the fish farm in the vicinity of Kurukshetra (Sultan Fish Farm, Village Butana, Nilokheri, Kurukshetra, India) and were Acclimated for 15 days. Two experiments (Table 1) were conducted to evaluate the effect of medicinal plant Mentha piperita (Peppermint) with or without probiotic bacterium Bacillus coagulans on growth performance and associated nutritional physiology of Catla catla in the Aquaculture Research Unit, Department of Zoology, Kurukshetra University, Kurukshetra.
Peppermint leaves (as leaves contain its active biological components) were collected from its natural habitat and identified properly. Leaves were dried for three to four days initially in the shade and then in an oven for 55 °C; thereafter, they were crushed into powder form using a pestle and mortar. Uniform powdered form of peppermint leaves was obtained by using a fine-meshed sieve and thoroughly mixed directly with basal diet. Basal diet was prepared by using groundnut oil cake (650 g), rice bran (32 g), duckweed (266 g), wheat flour (32 g), and mineral mixture (10 g). For Experiment 1, five isonitrogenous and isocaloric diets (C1, P1, P2, P3, P4) of 40% protein content were prepared (Table 2). Basal diet served as control. In Diet P1 to P4, Peppermint was added at 2 g Kg−1, 4 g Kg−1, 6 g Kg−1, and 8 g Kg−1 of feed, respectively. For Experiment 2, five isonitrogenous and isocaloric diets (C2, P1, PP2, PP3, PP4) of 40% protein content were prepared (Table 2). Control (C2) diet was prepared by adding probiotics Bacillus coagulans at the rate of 3000 CFU g−1 of feed to basal diet. In Diet PP1 to PP4, incorporation of probiotics Bacillus coagulans at the rate of 3000 CFU g−1 of feed along with peppermint was done at 2 g Kg−1, 4 g Kg−1, 6 g Kg−1, and 8 g Kg−1 of feed, respectively. All ingredients were thoroughly grounded, sieved, mixed, and then followed by preparation of dough homogeneously. Feed were prepared in the form of pellets by using a pelletizer. Feed was then oven-dried at the temperature of 60 ° C and stored in air tight containers. Probiotics-supplemented diet was stored at 4 °C to maintain it contamination-free.
Note: All values are mean ± S.E. of mean. Values with different superscript in the same row are significantly (P < 0.05) different (Tukey’s honest test)
The experiment was conducted in the Aquaculture Research Unit, Department of Zoology, Kurukshetra University, Kurukshetra. The Acclimated fingerlings were distributed in different aquaria (60 × 30 × 30 cm)/plastic tubs (50 L capacity) containing 10 fingerlings each under laboratory conditions (24 ± 1 °C). All groups of fishes in both experiments were fed daily at 3% BW in 2 installments at 10:00 and 17:00 h for 90 days provided 50–60% exchange of dechlorinated water daily along with constant aeration. Uneaten food was collected after 4 h of feeding for further analysis. Every individual fingerling was weighed at the start of the experiment, after every 15 days, and at the end of the experiment for the assessment of growth performance parameters.
Fecal matter was also collected daily and then oven-dried for determination of apparent protein digestibility with the help of chromic oxide (Cr2O3) as a digestibility marker according to Cho, Slinger, and Bayley (1982). Proximate analysis was performed following AOAC (1995). In proximate analysis, moisture content (evaporation in hot air oven at 95 °C for 48 h), crude protein (by Kjeldahl method), crude fat (by using petroleum ether in Soxhlet apparatus), and ash content (by incineration in muffle furnace at 550 °C for 4 h) were analyzed for both carcass and for feed. Crude fiber (by acid/alkali digestion method) was also analyzed for fish feed. NFE was estimated by subtraction method; NFE = 100 − (crude protein + fat + moisture).
During experimentation, at every 15th day, water quality parameters likewise temperature, pH, dissolved oxygen (DO), chloride, calcium, total alkalinity, electrical conductivity, and total hardness were analyzed following APHA (1998) to check the effect of feed on water characteristics.
At the end of 90 days feeding trials, fishes were offered the same diet and were allowed for 2 h to consume the feed. After 2 h, excess of feed was removed and fixed levels of water of all aquaria were maintained for experiments. After that, water samples from each aquarium were collected at 2-h intervals to evaluate the excretory levels of total ammonia (N-NH4) and reactive orthophosphate (o-PO4) following APHA (1998). The quantity of nitrogen and phosphate excreted by fish in holding water were calculated as follows:
where (N ‐ NH4/o ‐ PO4)0 and ( N ‐ NH4/o ‐ PO4)120 = Concentration at times 0 and 120 min (2 h) post feeding.a = amount of holding water (L) in which fishes were kept.
Various growth parameters were calculated for evaluating dietary performances and nutritional indices (Garg, Bhatnagar, Kalla, and Johal, 2002).
At the end of the feeding trial, two fingerlings from each treatment were taken, kept on ice tray, and their intestines were removed which was further processed for the determination of different intestinal enzyme activities such as protease (Walter, 1984), cellulase (Sadasivam and Manickam, 1996), and amylase (Sawhney and Singh, 2000).
Blood sample was collected from 5–6 fish from each treatment (fish were anesthetized using MS 222, Sigma-Aldrich) so as to pool the blood for the immunological, serological, and hematological diagnosis by caudal vein/heart puncture using a heparinized syringe, which was previously rinsed with EDTA solution and transferred immediately to an Eppendorf tube.
The collected blood samples were used for the estimation of total leucocyte count (TLC) and total erythrocyte count (TEC) with the help of hemocytometer using a Neubauer’s counting chamber following Dacie and Lewis (1963).
The phagocytic assay was performed according to the method of Siwicki, Anderson, and Rumsey (1994) and Park and Jeong (1996) with a slight modification. Blood samples were collected for determination of the phagocytic cells and phagocytosed bacteria for each treatment. Phagocytic activity (PA) and phagocytic index (PI) was determined by enumerating 100 phagocytes per slide under a microscope, and the average of three slides was calculated:
Phagocytic activity (i.e., percentage of cell with engulfed bacteria) = Number of phagocytic cells with engulfed bacteria/Number of phagocytic cells × 100.
Phagocytic index (i.e., number of engulfed bacteria per cell) = Number of engulfed bacteria/Number of phagocytic cells.
The oxygen radical production by blood phagocytes during respiratory burst activity was measured through Nitroblue tetrazolium (NBT) assay using spectrophotometer at 540 nm as described by Anderson and Siwicki (1995).
Serum from blood samples of each treatment was collected for serum bactericidal activity (Kajita, Sakai, Atsuta, and Kobayashi, 1990). The number of viable bacteria was calculated by counting the colonies from the resultant incubated mixture on TSA plates in duplicate (two plates per sample) after 24-h incubation. Less bacterial count will show better serum bactericidal activity.
The serum protein was determined according to Gornall, Bardawill, and David (1949). The blood samples were taken in a test tube which was not heparinized and allowed to stay until the clotted blood settled down. The serum was then transferred into another test tube. The serum was centrifuged for 10 min at 3000 rpm. After that, 0.1 ml of serum was treated with 5 ml of Gornal reagent (1.5 g CuSO4.5H2O, 6 g KNaC4H4O6, 500 ml H2O, 300 ml of 10% NaOH) and was shaken for 1 min. The absorbance of the mixture was determined spectrophotometrically at 540 nm, and the serum protein values were deduced by using a standard curve.
From each treatment, 10 fishes were challenged with A. hydrophila after feeding trial of 90 days (Austin, Stuckey, Robertson, Effendi, and Griffith, 1995). Percent survival was measured for 10 days based on the observation that mortality reached its elevation after 1 week (Sahoo, De, Ghosh, and Maitra,1998) and relative percentage survival (RPS) was calculated by the following formula (Ellis, 1988)
Kaplan-Meier survivorship curve was also constructed on MS Excel to show the pattern of fingerlings fed on different supplemented diets of M. piperita with and without probiotic in both experiments during challenge trial with the fish pathogenic bacterium, A. hydrophila, for ten days (Kaplan and Meier, 1958).
Data were subjected to one way ANOVA followed by Tukey’s honest test to test the significant differences between different dietary treatments (Tukey, 1977). Statistical significance was settled at a probability value of P < 0.05. All statistics were performed using SPSS Version 18.0.
Results
The growth parameters such as weight gain (WG), specific growth rate (SGR), protein efficiency ratio (PER), gross conversion efficiency (GCE), feed conversion ratio (FCR), and apparent protein digestibility (APD) of fish fed with a distinctive level of peppermint-supplemented diets are shown in Table 3. ANOVA followed by Tukey’s honest test revealed that WG, SGR, PER, GCE, and APD values were significantly (P < 0.05) high in all treatments in comparison to control with the highest value in dietary treatment P3. While FCR values were significantly low in all the treatments in comparison to control.
Weight gain (g) = W2 − W1; Growth % gain in BW = (W2 − W1)/W1 × 100; Growth per day in % BW = {2(W2 − W1)/t(W1 + W2)} × 100; SGR = (ln W2 − ln W1)/t × 100; FCR = Feed Offered (Dry wt.) (g)/Body weight gain (Wet wt.) (g); GCE = Body weight gain (Wet wt.) (g)/Feed Offered (Dry wt.) (g); PER = Wet Weight Gain (g)/Crude protein fed (%)
Where, W1 = Initial Weight (g), W2 = Final Weight (g), Duration of experiment (No. of days)
Note: All values are mean ± S.E. of mean. Values with different superscript in the same row are significantly (P < 0.05) different (Tukey’s honest test)
The WG, SGR, PER, GCE, FCR, and APD of fish fed with different inclusion levels of peppermint-supplemented diets with probiotic bacterium Bacillus coagulans are shown in Table 4. Significant increase in the values of these parameters was realized in treatment PP3 for this experiment. Significantly, low FCR values were recognized in fishes fed on a diet containing 6 g kg−1 of M. piperita along with probiotic bacterium Bacillus coagulans at the rate of (@) 3000 CFU g−1 in comparison to other treatments and control.
Weight gain (g) = W2 − W1; Growth % gain in BW = (W2 − W1)/W1 × 100; Growth per day in % BW = {2(W2 − W1)/t(W1 + W2)} × 100; SGR = (ln W2 − ln W1)/t × 100; FCR = Feed Offered (Dry wt.) (g)/Body weight gain (Wet wt.) (g); GCE = Body weight gain (Wet wt.) (g)/Feed Offered (Dry wt.) (g); PER = Wet Weight Gain (g)/Crude protein fed (%)
Where, W1 = Initial Weight (g), W2 = Final Weight (g), Duration of experiment (No. of days)
Note: All values are mean ± S.E. of mean. Values with different superscript in the same row are significantly (P < 0.05) different (Tukey’s honest test)
Specific protease, amylase, and cellulase activities were significantly high in those fingerlings whose diet was supplemented with M. piperita in comparison to control with highest value in dietary treatment P3.
After completion of the experiment, the increase of intestinal enzymes activity of fishes fed on different peppermint-supplemented diets along with probiotic bacterium Bacillus coagulans as comparison to control diet (Table 4) was clearly examined. Significantly (P < 0.05) higher intestinal enzymes activity was revealed in treatment PP3 in this experiment.
No significant variations were observed in water quality parameters. These parameters do not show any definitive trend with varying inclusion of peppermint in supplemented diet (Table 5). However, significantly (P < 0.05) low values of total ammonia excretion (454.33 ± 2.18) and reactive orthophosphate production (290.67 ± 1.20) were recognized in fish fed on peppermint-supplemented diet @ 6 g Kg−1 (treatment P3) compared to all other treatments in the first experiment.
Note: All values are mean ± S.E. of mean. Values with different superscript in the same row are significantly (P < 0.05) different (Tukey’s honest test)
Water quality parameters showed insignificant variations among all treatments. These parameters do not follow any specific trend with different diets containing different inclusion level of peppermint with probiotic bacterium Bacillus coagulans (Table 5). Despite this, PP3-treated fingerlings showed significantly (P < 0.05) low values of ammonia excretion (363.33 ± 2.03) and orthophosphate production (206.67 ± 1.76) than all other treatments.
After 90 days of feeding trial, final carcass composition of C. catla fingerlings of all treatments were analyzed and presented in Table 6. In this experiment, the carcass composition of test animals containing peppermint showed a significant increase in carcass protein, carcass lipid, and total gross energy in comparison to control fingerlings. Treatment P3-fed group fingerlings showed a significant increase in carcass protein (15.93 ± 0.03). Lipid content (6.99 ± 0.03) and gross energy (8.79 ± 0.01) were found to be highest in fishes of treatment P3.
Note: All values are mean ± S.E. of mean. Values with different superscript in the same row are significantly (P < 0.05) different (Tukey’s honest test)
In this experiment, the carcass composition of fingerlings fed on different incorporation levels of peppermint with probiotic bacterium Bacillus coagulans was analyzed and given in Table 6. Significantly highest carcass protein (16.14 ± 0.04) was observed in fingerlings of PP3 followed by PP2 (15.91 ± 0.02)-fed group fingerlings in the second experiment. Lipid values (7.00 ± 0.03) were significantly high in treatment PP3 followed by treatment PP4 (6.95 ± 0.02) and gross energy was also observed significantly higher in treatment PP3 followed by treatment PP2 fed group fingerlings.
The total erythrocytes were significantly (P < 0.05) higher in fishes fed on diet P3 (Fig. 1a). The total leucocytes were also significantly (P < 0.05) high in fishes fed on diet P3 (42.67 ± 0.88) than other treatments and control (Fig. 1b).
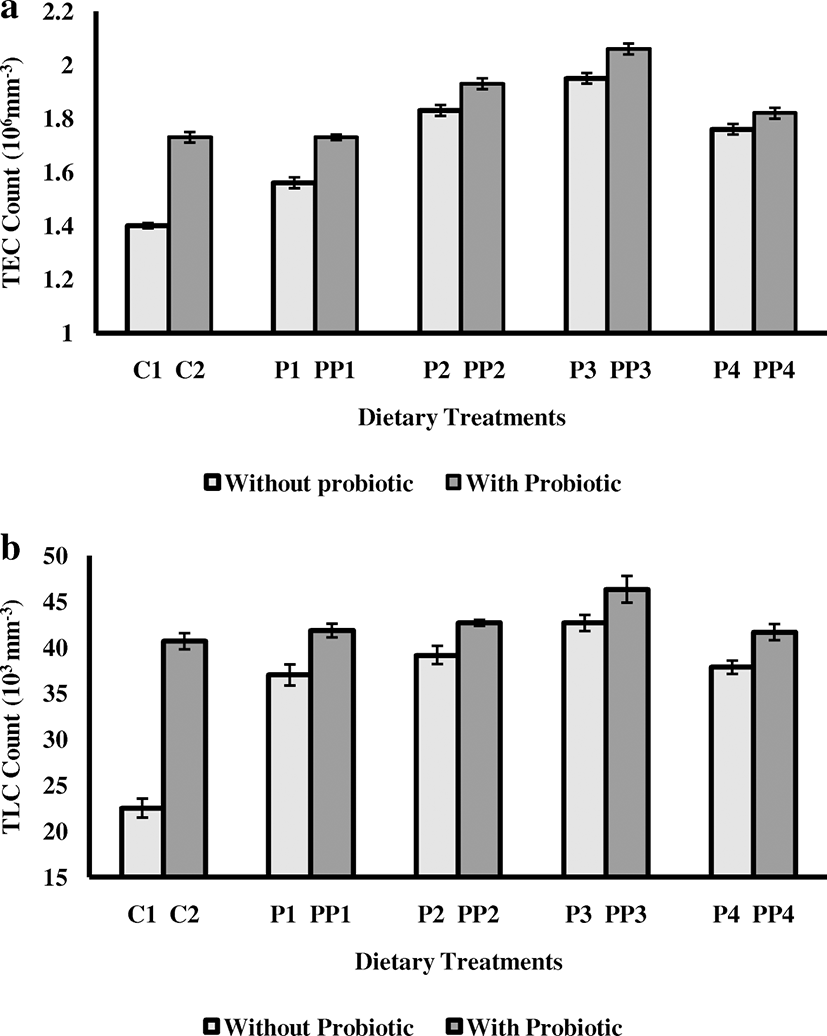
In this experiment, the values of total erythrocytes and leucocytes of fingerlings fed on different diets containing different inclusion level of peppermint along with probiotic bacterium are shown in Fig. 1a, b. The total erythrocytes (2.06 ± 0.02) and total leucocytes count (46.33 ± 1.45) of treatment PP3-fed group fingerlings depicted significant (P < 0.05) increase in comparison to other treatments and control.
The phagocytic activity and phagocytic indices of fish fed on a peppermint-supplemented diet after 90 days were significantly higher in treatment P3 (M. piperita @ 6 g Kg−1) followed by P2 (M. piperita @ 4 g Kg−1), P1 (M. piperita @ 4 g Kg−1), P4 (M. piperita @ 8 g Kg−1), and control (Table 7).
Note: All values are mean ± S.E. of mean. Values with different superscript in the same row are significantly (P < 0.05) different (Tukey’s honest test)
The phagocytic activity (82.09 ± 0.83) and phagocytic index (2.96 ± 0.01) of fingerlings fed on peppermint along with probiotics at 6 g Kg−1 (treatment PP3) showed significant (P < 0.05) increase than all other treatments in the second experiment (Table 7).
In this experiment, respiratory burst activity of treatment P3 (0.84 ± 0.01) followed by treatment P2 (0.71 ± 0.010), treatment P4 (0.63 ± 0.01), and P1 (0.53 ± 0.01) was significantly (P < 0.05) higher than control treatment (0.48 ± 0.01) (Table 7).
Respiratory burst activity of fingerlings fed on different inclusion levels of peppermint with probiotics was analyzed in which treatment PP3-fed group fingerlings exhibited significantly (P < 0.05) higher values of respiratory burst activity in comparison with fingerlings of all other treatments in the second experiment (Table 7).
Serum Bactericidal Activity is a measure of total bacterial colony count (103 CFU ml−1). The respective total bacterial count of fish serum against pathogenic strain fed on different diets such as P1, P2, P3, and P4 are 4.88 ± 0.05, 4.62 ± 0.03, 4.03 ± 0.15, and 4.36 ± 0.08 (103 CFU ml−1) indicating significantly improved activity in fingerlings of group P3. The value in the serum of fishes in the control (C1) was 5.10 ± 0.08 (Table 7).
Serum Bactericidal Activity of fingerlings fed on peppermint at 6 g Kg−1 along with probiotics showed significantly (P < 0.05) better response than other treatments in the second experiment as viable bacterial colony count is lowest compared to the rest of treatments including control (Table 7).
Total serum protein (μg ml−1) in this experiment was found significantly (P < 0.05) higher in the fingerlings fed on peppermint-supplemented diet at 6 g Kg−1(3.94 ± 0.01) followed by 4 g Kg−1 (3.59 ± 0.02), 8 g Kg−1(3.51 ± 0.01), and 2 g Kg−1 (3.42 ± 0.02) (Table 7).
In this experiment, significantly (P < 0.05) higher total serum protein was detected in fingerlings fed on peppermint-supplemented diet along with probiotics at 6 g Kg−1. The respective total serum protein values (μg ml−1) of fingerlings of different treatments such as P1, P2, P3, and P4 are 3.42 ± 0.02, 3.59 ± 0.02, 3.94 ± 0.01, and 3.51 ± 0.01, and in the control the value is 3.38 ± 0.01.
Post challenge relative survival rates of fingerlings fed on peppermint-supplemented dietary treatment P3 (67.93 ± 6.62) were recognized significantly (P < 0.05) high in comparison to other dietary treatments such as P2 (50.95 ± 4.97) followed by P4 (33.96 ± 3.31) and P1 (16.98 ± 1.65), while the fingerlings fed on control diet showed the highest mortality (60.00 ± 5.77) and fingerlings of treatment P3 exhibited the lowest mortality (20.00 ± 5.77) (Fig. 2a). The Kaplan-Meier plots for survival of fish in Experiment 1 after challenge trial are presented in Fig. 3 a revealing that there is a significant difference between the survival trends in each treatment. The initial mortality was observed in treatment control, P1 and P4, while in treatment P2 and P3 mortality was observed after 3 days and 6 days, respectively.
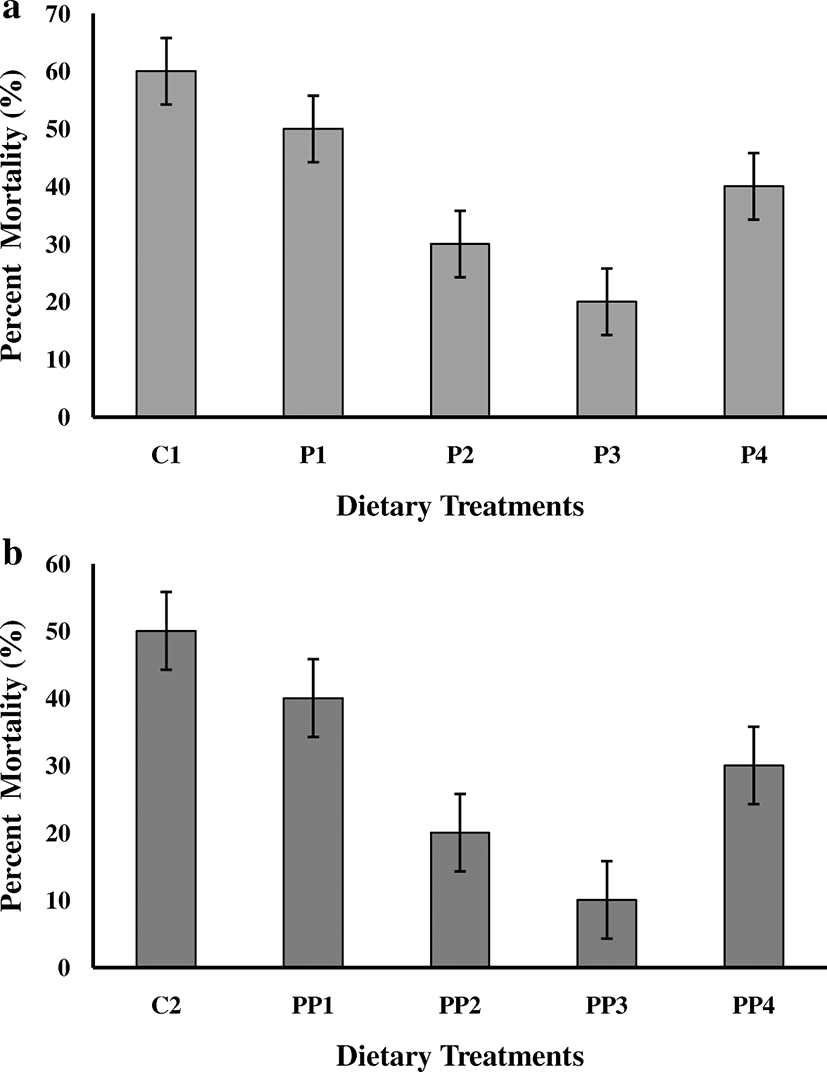
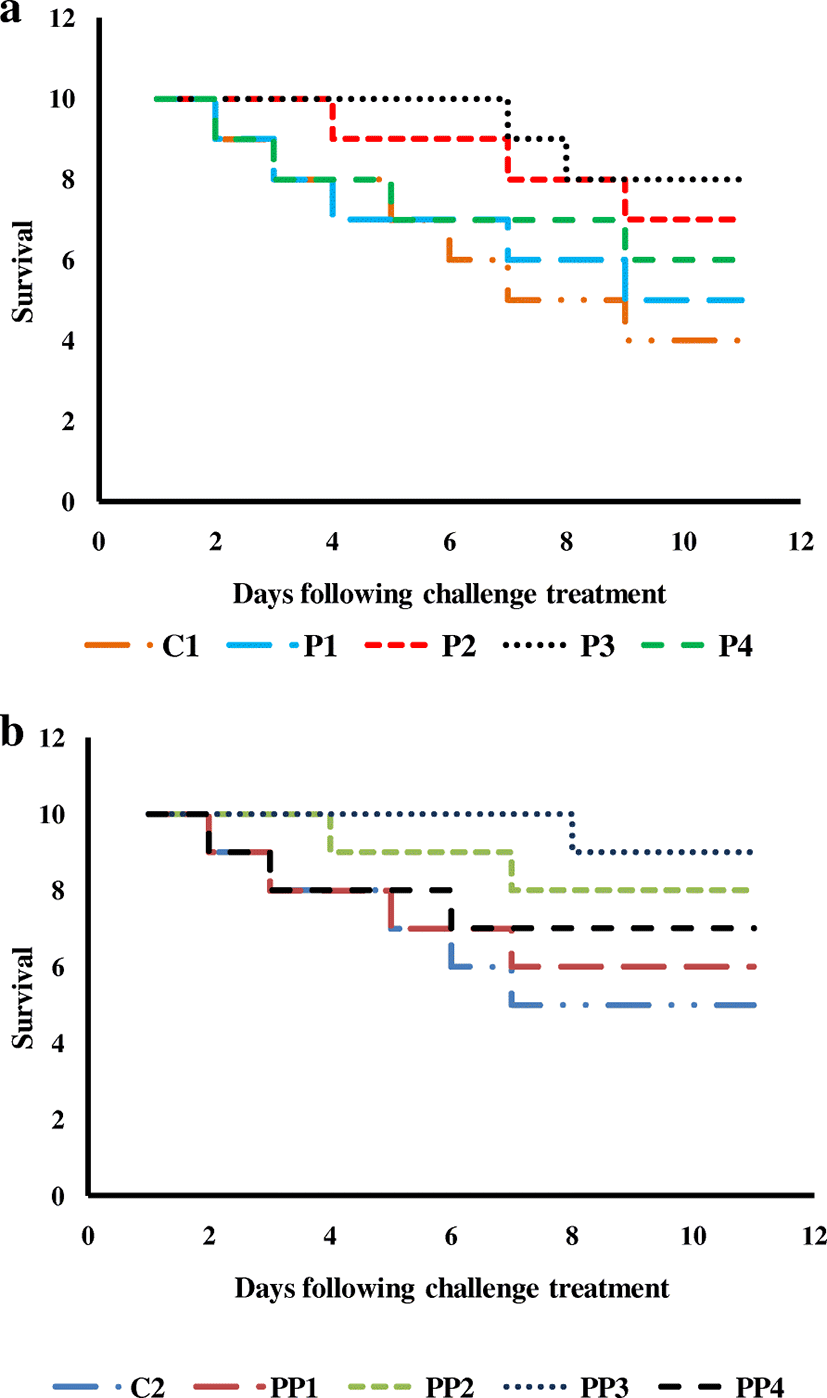
Post challenge relative survival rates of fingerlings fed on peppermint-supplemented dietary treatment along with probiotics at 6 g Kg−1 (82.22 ± 9.68) were examined significantly (P < 0.05) high in contrast with other dietary treatments such as PP2 (56.11 ± 16.95) followed by PP4 (41.11 ± 4.84) and PP1 (20.55 ± 2.42), while the fingerlings fed on the control diet revealed the highest mortality (50.00 ± 5.77) and fingerlings of treatment PP3 exhibited the lowest mortality (10.00 ± 5.77) (Fig. 2b). The Kaplan-Meier plots for survival of fish in Experiment 2 after challenge trial are presented in Fig. 3 b revealing that there is a significant difference between the survival trends in each treatment. The initial mortality was observed in treatment control, P1 and P4, while in treatment PP2 and PP3 mortality was observed after 3 days and 7 days, respectively.
Discussion
Plant extracts have been reported to various prospective as a growth stimulator in aquatic creatures (Citarasu, Babu, Sekar, and Petermarian, 2002). The present study concedes that C. catla when fed on M. piperita-supplemented diets significantly (P < 0.05) increased the growth performance along with a better immune response. M. piperita has been acknowledged as appetite accelerator because it promotes daily feed consumption (Nobakht and Mehmannavaz, 2010), and subsequently, low values of FCR ultimately led to high weight gain, high growth % in body weight, high SGR, high GCE in M. piperita-fed group fishes. The reason might be attributed that M. piperita could upgrade the digestibility and proper utilization of nutrition leading to protein synthesis which impacted ultimately on growth performance. The results in first experiment of our study are thus in accordance with the study of Talpur (2014) and Adel, Amiri, Zorriehzahra, Nematolahi, and Esteban (2015a) who fed M. piperita diets to Asian seabass, Lates calcarifer (Bloch), for 8 weeks and fry Caspian white fish for 4 weeks, respectively, and observed significantly higher growth rate in test fishes in comparison to control.
Crude protein and gross energy of fishes fed on a peppermint-supplemented diet at 6 g Kg−1 (treatment P3) were found significantly high when compared with other treatments. The NFE in test fish may illustrate that they do not accumulate carbohydrate in their tissue as a major part was used as a reservoir of energy. The NFE content recognized during proximate analysis might be developed from basic sources such as glycoproteins and glycolipids. Our results for NFE content in fishes during proximate analysis are in thus accordance with the findings by Bob-Manuel and Alfred-Ockiy (2011) and Orire and Sadiku (2014) in which they observed NFE (%) content in the range of 16.33–38.11 and 0.17–8.66, respectively. Peppermint promotes the synthesis of protein in high amounts and thus high protein accumulation. Enzymatic activity results of the present study concluded that peppermint can upgrade intestinal enzymes and microbiota of fish. Significant differences were observed in enzymatic activities (protease, amylase, cellulose) of treated groups as compared to control. On the contrary, no significant (P < 0.05) difference was observed in protease activity but significant (P < 0.05) difference was recorded for amylase activity of Caspian brown trout when they were fed on a peppermint-supplemented diet by Adel, Safari, Pourgholam, Zorriehzahra, and Esteban (2015b). The reason might be due to different feeding habits of Caspian brown trout in their study and C. catla in the present study. As vegetable protein is difficult to digest in non-carnivorous fish, it requires more proteolytic enzyme activity for utilizing the protein present in the diet. In our study, total leucocytes and total erythrocytes count were also increased significantly (P < 0.05) in M. piperita-supplemented diet-fed group which is similar to the findings of Adel et al. (2015 a, b) and Nya and Austin (2009) who obtained increased levels of RBC and WBC when Caspian brown trout were fed on different peppermint-supplemented diets and Oncorhynchus mykiss fed on different ginger-supplemented diets. However, post challenge data reveal that WBC count of blood increased and RBC count of blood decreased, as the increase of WBC count is an important phenomenon of defense when any foreign microorganism causes any infection. Our results coincide with the results of Talpur and Ikhwanuddin (2012) and of Talpur, Ikhwanuddin, and Bolong (2013) who also realized the increase count of WBC when L. calcifer were fed on garlic- and ginger-supplemented diet, and Talpur (2014) determined similar results when L. calcifer were fed on different M. piperita-supplemented diets.
Phagocytosis and respiratory burst activity is acknowledged as a predominant mechanism countering bacterial defense. Mentha species have phenomenal antimicrobial and antioxidant properties as reported by Mimica-Dukic, Bozin, Sokovic, Mihajlovic, and Matavulj (2003) and Mahboubi and Haghi (2008). The result of the present study manifested high phagocytic activity (Table 7) in peppermint-supplemented diet which clearly revealed that supplementation of M. piperita in diet enhanced the non-specific immune response significantly (P < 0.05) which is related to the results of Abasali and Mohamad (2010) and Talpur (2014) who reported the remarkably increased activity of phagocytosis in C. carpio and Lates calcifer after feeding on M. piperita-supplemented diets. The superoxide anion production can be assessed by Nitroblue tetrazolium assay (NBT) by calibrating free oxygen radicals (Anderson and Siwicki, 1995). The outcome of the present study illustrated that respiratory burst activity was observed to be significantly (P < 0.05) higher in treatment P3 fed group fingerlings containing M. piperita at 6 g Kg−1 of feed.
A crucial immune response which is concerned with the conquering of pathogenic microbes is serum bactericidal activity (Ellis, 1999). Serum bactericidal activity of all treated fed group fingerlings showed significant increase than the control treatment. Remarkably, better serum bactericidal activity was observed in treatment P3 (containing peppermint 6 g Kg−1 of feed), as pathogenic bacteria plate count was found to be lesser in this treatment in comparison to others. Our results are constant with the results of Nya and Austin (2009) who achieved elevated serum bactericidal activity when Oncorhynchus mykiss were fed on ginger-supplemented diets; Abasali and Mohamad (2010) who calculated the bacterial count and observed the increased serum bactericidal activity after feeding on M. piperita in C. carpio; Talpur and Ikhwanuddin (2012), Talpur et al. (2013), and Talpur and Ikhwanuddin (2013), who assessed the increased serum bactericidal activity after feeding on garlic, neem leaf and ginger, respectively, in L. calcifer; and Talpur (2014) who found the significantly (P < 0.05) high serum bactericidal activity in L. calcifer after feeding on M. piperita. This improved serum bactericidal activity in these studies and also in the present study clearly reveal better immune response which might be attributable to the existence of bioactive components such as phenol, tannins, and flavanoids (Pramila et al., 2012) in M. piperita leaves which are known for antimicrobial and anti-inflammatory properties (de Sousa Araújo, Alencar, de Amorim, and de Albuquerque, 2008). The serum protein level was also monitored and significantly (P < 0.05) higher values were observed in fingerlings fed on M. piperita at 6 g Kg−1 than their respective control treatment fingerlings which resembled with the results of Sunitha et al. (2017) who observed significant increase in serum protein in Cyprinus carpio after feeding on Phyllanthus niruri. The values of serum protein reflect the capability of the liver for protein synthesis and signify renal functioning and blood osmolarity (Sunitha et al., 2017). Thus, high serum protein in treatment P3 clearly reveals better capability of protein synthesis and thus coinciding with high growth and high carcass protein; therefore, supplementation of M. piperita at 6 g Kg−1 appears to be optimum dose. Moreover, increasing level of serum protein in the group of fish fed on M. piperita-supplemented diets might be due to active components such as menthone, isomenthone, and 1, 8-cineole (Mimica-Dukic et al., 2003), which helps in the prevention of different infectious diseases leading to better immunity.
In the second experiment, probiotic bacterium Bacillus coagulans at 3000 CFU g−1 of feed was also incorporated along with different levels of M. piperita . The result showed high growth performance in terms of WG, SGR, PER, GCE, and APD with low FCR in group of fishes fed on diet PP3 (with M. piperita @ 6 g Kg−1of feed and B. coagulans @ 3000 CFU g−1). WBC and RBC count was significantly high along with high phagocytic activity, respiratory burst activity, and better serum bactericidal activity with low excretion of metabolites (ammonia excretion and reactive phosphate production) in group of fingerlings of treatment PP3 which further demonstrated that inclusion of M. piperita @ 6 g Kg−1 of diet is optimum for better growth performance, nutrient retention, and immunity of C. catla. On comparing the results of Experiment 1 and 2, it was observed that the growth performance, nutrient retention, and immunity parameter values were high for the respective groups of Experiment 2 with significantly higher value in treatment PP3 where fishes were fed on a diet containing M. piperita @ 6 g Kg−1 of diet and B. coagulans @ 3000 CFU g−1. This might be attributed to the reason that the presence of autochthonous probiotic bacterium supplemented in diet altered the microbial metabolism of fishes or might be due to the colonization of probiotic bacteria in the gut of fishes that expanded its exogenous enzyme production ultimately promoting the growth performance (Bhatnagar et al., 2012). It has been accounted that probiotic bacterium also provides the unsaturated fatty acid and vitamins involved in host nutrition (Sakata, 1990).
On comparison with TLC and TEC count in Experiment 1 and 2, TLC and TEC count increased with increasing dose of M. piperita up to the level of 6 g Kg−1 of diet and then count declined with further increasing the dose in the first experiment. It clearly revealed that M. piperita-supplemented diet @ 6 g Kg−1 of feed is optimum. However, in Experiment 2, the same trend was observed, but with the addition of probiotic along with M. piperita-supplemented diet, TLC and TEC count were increased more at each respective dose level (Fig. 1). This might be due to the reason that active compounds present in M. piperita and probiotic bacterium synergistically stimulated the humoral immunity at optimum level. Probiotic bacterium incorporated in diet inflected an immune response of fishes by depressing the pathogenic responses by enhancing leucocyte count which involved in phagocytic activity. Balcazar (2003) illustrated the incorporation of several probiotic bacterium strain (Bacillus and Vibrios sp.), decidedly impacted the development and survival of adolescents of white shrimp, and displayed a defensive impact against Vibrio harveyi because of an incitement of immune system by expanding phagocytic activity and antiviral activity. Moreover, Nikoskelainen, Ouwehand, Bylund, and Salminen (2003) demonstrated that supplementation of Lactobacillus rhamnosus @ of 105 CFU g−1 of feed accelerated the respiratory burst activity in Oncorhynchus mykiss. Significant high phagocytic activity, phagocytic index, respiratory burst activity, and better serum bactericidal activity of probiotic-supplemented fingerlings in our study also supported this view as it enhanced the production of phagocytes during invasive attack of pathogen by defense mechanism. In addition to this, supplementation of M. piperita in the diet also improved feed consumption expanding the intestinal activity with proper utilization of feed which resulted in synergistic effects on growth performance and immunomodulatory responses in Catla catla. The challenge trial acknowledged that oral administration of M. piperita-supplemented diet along with probiotic bacterium boosted the defiance of C. catla from pathogenic infection and coincided with the results of Bhatnagar and Lamba (2017) in which B. cereus was when incorporated in diets at different inclusion level for Cirrhinus mrigala showed the high survival of probiotic-fed group fishes as compared to the control diet fishes.
The Kaplan-Meier assessment is the simplest way to figure out the survival over time despite all these complications related with subjects or circumstances. This survival pattern clearly indicated that treatment P3 and PP3 showed high survival while high mortality was detected in fingerlings of control treatment in both experiments conceding the immunostimulating effects of dietary supplementation of M. piperita as well as of probiotics.
Conclusion
The results of the present study signified that use of M. piperita-supplemented diet can significantly improve growth and immunity in C. catla without compromising water quality, and further improvement can be obtained when such diets are further supplemented with autochthonous probiotic bacterium. Since, to the knowledge of authors, no work has been conducted yet on the synergistic effect of autochthonous probiotic bacterium- and plant-supplemented diet, so it can make a better approach towards detailing of an ideal diet by using supplementation of different plant extract along with other autochthonous probiotics for better growth performance and immunity of fish that can prompt sustainable aquaculture.