Background
Lactococcus garvieae is a gram-positive and non-moving bacteria, and pathogen of lactococcosis, and affects much wild and cultured fish; it is an emerging zoonotic pathogen that causes serious infections in humans and animals (Vendrell et al. 2006). Lactococcosis is a systemic disease in marine fish including tilapia, sea bass, eel, and yellowfish and is now recognized as one of the most important diseases of the rainbow trout in aquaculture (Austin and Austin 2007, 2016; Meyburgh et al. 2017; Chapela et al. 2018). Several reports are presented of lactococcosis occurrence in marine fish, brackish water, and freshwater, especially when water temperature increases over 15 °C (Austin and Austin, 2007). The first occurrence of lactococcosis in rainbow trout was reported in Spain in 1988 (Ghittino and Prearo 1992). This pathogen has caused serious economic losses in the cultivation of marine and freshwater fishes (Vendrell et al. 2006; Wang et al. 2007; López et al. 2015; Meyburgh et al. 2017). Farming rainbow trout fish has been developed during the last decades in different suitable areas of Iran, but unfortunately, some diseases and health problems such as lactococcosis have caused a lot of damage to the fish culture industry and damages to tens of millions per year (Soltani et al. 2016). The L. garvieae was first isolated in Iran from a rainbow trout farm in Fars province (Soltani et al. 2005). The incidence of lactococcosis in Iran is mainly in the livestock stage, which may cause damage to fish farms between 5 and 75%, and the major losses occur in the fattening stages, so the damages are significant and the agents of this disease can also be transmitted to humans causing endocarditis, cholecystitis, and diskospondylitis, so the human health debate can also be important (Wang et al. 2007; Chan et al. 2011; Kim et al. 2013; Soltani et al. 2015; Gauthier 2015; Meyburgh et al. 2017). Environmental factors, hosts, and pathogens play a role in the development and spread of this disease in rainbow trout farms, so attention to bacterial strains of the disease and its various reservoirs is very important in controlling the disease and preventing it (Soltani et al. 2009). Considering that L. garvieae is the primary cause of mortality and risk factor in the rainbow trout culture industry during hot seasons; therefore, the purpose of this study was to identify and detect strains isolated from rainbow trout suspected of having L. garvieae using biochemical characteristics and PCR and determination of the degree of severity of isolated strains.
Methods
During the fall of 2014, the bacterial strains of L. garvieae were obtained from suspected fish of Koohdasht, Kohkilooieh, and Boyerahmad province, the southwest of Iran. A total of 30 rainbow trouts with a weight range of 100–200 g were collected from suspicious farms. Suspect fish were transferred to the laboratory of the Department of Aquatic Animal Health, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, alive.
Bacterial culture was performed on suspected fish with symptoms of hemorrhage, exophthalmia, septicemia, and melanosis. The kidney and brain samples were streaked onto sterile conditions on blood agar (Merck, Darmstadt, Germany) and trypticase soy agar (TSB, Biokar Diagnostics, Zac de Ther, France) medium, and incubated at 25 °C for 48 h. Single colonies from plates with pure culture growth were re-streaked on the BHIA (Brain Heart Infusion Agar; Merck, Darmstadt, Germany) medium to obtain pure isolates.
Gram-positive cocci and catalase-negative pure colonies were subjected to gram staining. The biochemical properties of the isolates were determined according to the method recommended by Austin and Austin (2007) and Soltani et al. (2005). Hemolysis test of sheep red blood cells was performed at 25 and 37 °C. The Lactococcus isolates were tested for bacterial growth potentials at different temperatures (10, 37, and 45 °C) and at different pH (5–5.9), catalase, oxidase, VP (Voges-Proskauer) reaction, hydrolysis, different sugar consumption, oxidation and fermentation of glucose (o/f), production of indole and H2S, and movement test in the SIM (sulfide-indole-motility; Merck, Darmstadt, Germany) medium. All of these tests were read after 24 h of incubation at 30 °C.
To confirm the diagnosis of the disease, sorbitol-positive isolates with similar characteristics of L. garvieae were used for PCR studies. For this, colony PCR was performed using an amplicon extraction kit (CinnaGen, Tehran, Iran) based on the kit’s instructions, and the DNA quality extracted by electrophoresis (NanoDrop Spectrophotometer, SPX2, Eppendorf, Germany) on a 1.5% agarose gel (CinnaGen, Tehran, Iran) was investigated. PCR test was done based on the method recommended by Woodman (2008) using PLG-F (5-CATAACAATGAGAATCGC-3) and PLG-R (5-GCACCCTCGCGGGTTG-3) primer sequences for amplification of 16S rDNA sequence and observation of a band of 1100 bp. To perform the PCR reaction, 10 μl of Master Mix (Amplicon kit) was added to the 0.2-ml microtube, followed by 0.5 μl of each primer. Then, a pure colony of bacteria cultured with sterile applicator was poured into the microtube under sterile conditions, and 6 μl distilled water was added to it. The PCR steps were performed using ThermoCycler machine (Eppendorf, USA) with initial denaturation for 5 min (95 °C), denaturation for 1 min (94 °C), annealing for 45 s (58 °C), elongation for 1 min (72 °C), then repeat steps 2–4 to 30 cycles, and finally the final elongation step for 5 min (72 °C). The PCR product was electrophoresed with agarose gel 1.5%, and after staining with a safe stain (CinnaGen, Tehran, Iran), the gel was photographed using the Gel Documentation (Gel doc, 6454, Mina Tajhiz Pars, Iran).
The antibiotic test was used to determine the susceptibility of isolated bacteria from common antibiotics using standard disk diffusion (Bauer et al. 1966). For this purpose, antibiotics such as tetracycline, streptomycin, enrofloxacin, ciprofloxacin, lincospectin, and florfenicol were used. For this purpose, a bacterial concentration of 0.5 McFarland was prepared in a nutrient medium of Mueller-Hinton medium (Merck, Darmstadt, Germany) and then cultured on agar of Mueller-Hinton agar medium by the spread plate method which was cultured in five directions. Antibiotic disks were inserted at appropriate intervals. Plates were incubated at 37 °C for 24 h, and the inhibition halo diameters were measured.
Due to the fact that all bacteria isolated from fish have the same biochemical and molecular properties and there was no difference in their biochemical and molecular methods, the source bacteria contamination was detected on one strain, and to determine the bacterial severity, the conventional LD50 method by the technique of Reed and Muench (1938) and Probit software were used as follows:
In short, after bacterial culture in TSB medium for 48 h at 25 °C, bacterial isolation was performed using a centrifuge at 3000g for 15 min. The isolated bacteria were washed with sterile PBS (phosphate-buffered saline; Merck, Darmstadt, Germany). Using McFarland tubes and control by viable colony count, dilutions were sequentially based on 10 (104 to 10). The selection of concentrations was determined based on the initial pilot test. After the adaptation of rainbow trout (average weight 30 g) to culture conditions in the laboratory, the fish were infected with these dilutions. At first, fish of each treatment were anaesthetized by 2-phenoxyethanol (Merck, Darmstadt, Germany) at a concentration of 400 ppm in 1-l water and then 0.1 ml of each bacterial dilution injected intraperitoneally into 10 fishes in 3 replicates. To the control group, 0.1 ml of sterile PBS was injected. After injections of bacterial dilutions, the number of casualties was recorded up to 10 days after injection. To ensure the cause of the death of fish, the culture was performed on the kidneys and brain of the fish, and the injected bacterium was re-isolated.
The results of the study of bacterial severity were evaluated using Reed and Muench’s (1938) technique and Analysis Program Probit EPA software version 1.5. In this software, the medium lethal dose (LD50) is determined in a given time with an error rate of less than 0.05.
Results
The water temperature was recorded at 17 °C during the course of the disease. From the beginning of the mortality to the time of sampling (about a week), the proportion of mortality was close to 5% of the population. The sick fish showed behavioral symptoms including: lethargy, neglect, lack of nutrition, accumulation at the inlet and outlet, including irregular and sometimes rotational swiming and clinical signs of the disease including bilateral exophthalmia and hemorrhage in the eyes, darkening of the body color, cutaneous ulcers (in some cases) accompanied by bleeding in the dermal region of the abdominal and chest area, as well as anatomical symptoms including enlargement and color-change in the spleen, swelling, blurry and hemorrhage of the kidneys and the liver, increased abdominal fluid volume, and hemorrhages in visceral fats.
The bacterial culture results from the brain and kidney organs of the sick fish resulted in the isolation of non-moving gram-positive cocci and catalase-negative and oxidase-negative. These results were observed in almost all samples taken from different farms. The results of the sorbitol test showed that isolated strains were able to fertilize sorbitol glucose, which confirmed the L. garvieae species. Other results of biochemical properties of these isolates are presented in Table 1, so that in comparison with the properties of the isolates reported in the authoritative and official sources, all strains isolated from the fish are likely to have the same source and are classified in the Lactococcus garvieae species.
A/A− acid/acid no gas, F fermentation, v variable
The results of the antibiogram test showed that the isolated L. garvieae species had the highest susceptibility to florfenicol and lincospectin antibiotics and had no sensitivity to tetracycline, streptomycin, enrofloxacin, and cyrofloxacin (Table 2).
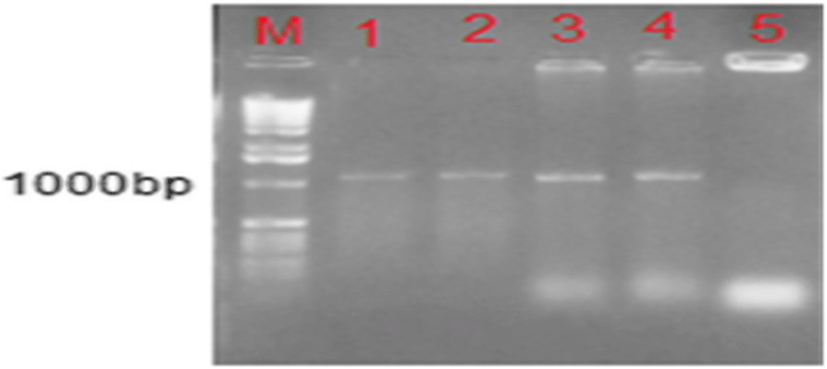
Molecular study (PCR) was performed on all isolated strains that were gram-positive coccidia and catalase-negative and sorbitol-positive. The results show that the expected bands of 1107 bp show 16s rDNA gene for L. garvieae in all isolates (9), which were ultimately confirmed by the detection of L. garvieae isolated from the sick fish (Fig. 1).
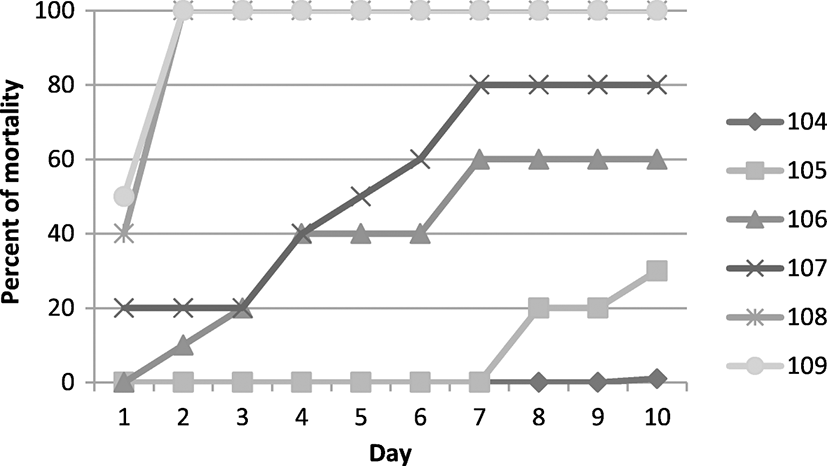
Considering the similarity of the strains examined and the probability of the disease spreading from a single source (possibly suspected fry fish), one of the isolates from the farm with the highest mortality rates was used to check the severity. The results of the bacterial severity study based on cumulative mortality after bacterial contamination (challenge) and using the method of Reed and Muench (1938) are presented in Fig. 2 and Table 3. The LD50 of 7.4 × 105 was calculated using Probit software.
|
Lethal dose |
10 days |
Low range |
Upper range |
|---|---|---|---|
|
LC10 |
1.2 × 104 |
3.84 × 104 |
6 × 104 |
|
LC50 |
4.7 × 105 |
1.1 × 105 |
1.7 × 106 |
|
LC75 |
3.1 × 106 |
9.4 × 105 |
2.3 × 107 |
|
LC90 |
1.7 × 107 |
4.1 × 106 |
3.8 × 108 |
Discussion
Lactococcosis is a devastating disease in the culture of salmonids. The first occurrence of lactococcosis in rainbow trout was reported in Spain in 1988. Since then, lactococcosis has been causing severe casualties in cultured rainbow trout in many parts of the world including Spain, Iran, Australia, South Africa, Japan, Korea, USA, Taiwan, Greece, England, Italy, France Portugal, Turkey, and Bulgaria (Didinen et al. 2014; Raissy et al. 2018; Baños et al. 2019). This disease has been recognized as one of the major problems in the culture of cold-water fish in Iran, especially in the last two decades. In recent years, this disease has caused serious damage to the culture industry of rainbow trout in some provinces, according to reports (Akhlaghi and Keshavarzi 2002).
The L. garvieae was first isolated in Iran from a rainbow trout farm in Fars province. The reported symptoms include irregular swimming, black body color, hemorrhagic septicemia, ascites, bilateral exophthalmia, cataract, and hemorrhage in the liver, spleen, kidney, brain, and intestine, and mortality rates in the farms varied from 40 to 20% (Soltani et al. 2005). The registered clinical symptoms in this study were similar to the symptoms mentioned in other studies in Iran and other parts of the world (Ghittino and Prearo 1992; Prieta 1993; Eldar et al. 1999; Vendrell et al. 2004, 2006). In this study, the bacteria isolated from all samples were of the same origin and all biochemical and molecular characteristics were the same. Therefore, a type of bacteria likely caused the mortality in the studied farms. By examining the source of fish supply in selected farms, this possibility was confirmed, but due to the fish in the farms examined were supplied from different sources and at different periods, it cannot be safely identified as the main source of infection. On the other hand, it is possible that this strain overcame other strains of the region due to higher severity and could be maintained due to resistance to common antibiotics in the region. The biochemical characteristics of the isolated bacteria showed that the tested species were L. garvieae. Despite the high compliance with biochemical factors, the species was not consistent with the results of Austin and Austin (2007) in the Voges-Proskauer reaction and the consumption of saccharose and salicin sugars. But the results of the study were consistent with the results of Soltani et al. (2008) and Sharifiyazdi et al. (2010) in these indices which show the similarity of this strain with strains isolated from other regions of Iran. So it confirms the hypothesis that this strain may be considered as a dominant species due to high antibiotic resistance and a high level of resistance in different regions of the country.
Despite the possibility of infecting fry fish in the aquaculture farms, it cannot be described as the main cause. Failure to observe the principles of biosecurity in farms creates the possibility of horizontal transmission of infection from a farm to another farm by water, birds, workers, visitors, and aquaculture equipment. There are reports that at specific times, a specific bacterial isolate is spread over a large area and replaces old and low severity strains due to its high resistance and severity (Kim et al. 2004). However, this indicator should be taken into account in the fight against this disease in the region and the design of the appropriate control method. While biochemical properties are a common method for identifying L. garvieae, due to frequent errors in diagnosis, the usefulness of this method has been questioned. Therefore, the use of molecular techniques is a suitable method for accurate and rapid identification of bacterial strains (Roach et al. 2006). In this study, type-specific primers were used for the identification of bacteria and the results of the PCR product showed that all strains isolated from samples were L. garvieae and were quite similar.
The isolated L. garvieae from this study was sensitive to fluorophenicol, lincospectin, gentamicin, and tylosin; had the most sensitivity to fluorophenicol; and showed no sensitivity to tetracycline, enrofloxacin, streptomycin, and ciprofloxacin antibodies. These findings were consistent with the results of Ravelo et al. (2001). In a study by Soltani et al. (2008), isolated L. garvieae was only susceptible to enrofloxacin and ampicillin. Sharifiyazdi et al. (2010) reported that the isolated L. garvieae was sensitive to erythromycin, sulfadiazine, and chloramphenicol antibiotics. Today, fluorophenicol, oxytetracycline, and enrofloxin antibiotics are the most commonly used on farms. Due to the inappropriate use of tetracycline and some other antibiotics, the complete resistance to them in fish has been created, which is probably due to the gene’s emergence of resistance to these antibiotics in pathogenic bacterial species. In a study by Kim et al. (2004), tet (S) and tet (M) resistant genes were found in L. garvieae isolated from fish. By examining the sampling sites, it was found that antibiotics that were found to be ineffective in the bacteria were used with high doses and prolonged periods of many occasions in the emergence of disease. It is possible that high resistance to these antibiotics is due to its high dosage and failure to complete treatment period and the unnecessary repetition of antibiotic therapy in the past years. On the other hand, the high sensitivity of L. garvieae to fluorophenicol confirms the value of this antibiotic in the fight against this disease, so the least resistance to this antibiotic in the pathogens was reported in the sources (Sharifiyazdi et al. 2010).
In this study, the LD50-isolated L. garvieae was determined to be 7.4 × 105 CFU/ml after 10 days of the challenge, indicating a very high severity of these bacteria in rainbow trout. In the study of Sharifiyazdi et al. (2010), LD50 of isolated L. garvieae was also estimated to be 6 × 105 CFU/ml, which showed a lower severity than the current study. In a similar study, Chen et al. (2002) reported that L. garvieae with a concentration of 100 × 108 × 108 resulted in 100% mortality and they reported a concentration of 1:106 × 1 CFU/ml as the LD50 of this bacterium in Mullet. Türe et al. (2014) estimated the LD50 of L. garvieae isolated from rainbow trout by 105 × 1.7 CFU/ml, which is a higher severity of bacteria in the present study. Lactococcosis is recognized as an acute septicemia disease, the clinical signs of this which are very similar in many species (Chen et al. 2002). Water temperature is an important factor in the spread of disease, and it has been reported that increasing the temperature during the summer months causes the severity of the disease to increases. Although the disease can occur in all ages according to species, age, and size of the fish, the severity of the disease and environmental stresses can vary from acute to chronic (Evans et al. 2009). Perhaps some differences in the LD50 reported by different researchers are due to the difference in water temperature, the size of tthe fish and fish species; however, in the present study, attempts were made to make all the cases according to standard protocols. The study of Soltani and Tarahomi (2008) showed that 20% of the gram-positive cocci isolated from Fars province farms were L. garvieae. In the research of Soltani et al. (2015), the relative abundance of Lactococcosis was estimated to be 86.36% in Lorestan province and this ratio was 85.71% in Fars province. Due to the relative frequency in these two provinces, a significant number of farms are involved in lactococcosis.
The occurrence of Lactococcosis in rainbow trout farms occurs mainly from late June to early November. At the time of the onset of the disease, the temperature of the water was 17 °C, which increased the chances of getting the disease by increasing the temperature in the heating season and decreasing the water flow.
A recent study has reported that lactococcosis is developing in Chahar Mahal Bakhtiari and Kohkiluyeh and Boyerahmad provinces, and they have estimated the risk factors for the spread of this disease that include the weakness of health management, the lack of consideration of risk factors such as the presence of bacterial reservoirs near fish farms, the pollution of water to human and urban sewage, and the lack of timely vaccination (Soltani et al. 2013). The results of Soltani et al. (2009) have shown that there is a lot of damage caused by lactococcosis in rainbow trout culture. On the other hand, given the fact that the disease is a zoonosis and the possibility of transmitting the disease to humans, it justifies the need for further attention. In such circumstances, the need to adopt practical and effective policies to combat the disease, such as vaccination and planning to eradicate the disease, felt more and more.
Conclusion
In general, according to current research, it can be concluded that the condition of lactococcosis in the studied area is not suitable, and despite the presence of disease, there is no proper action to control and prevent the disease. Unfortunately, isolated bacteria from the studied area have a very high severity compared to bacteria isolated from other regions of the country or other countries. Therefore, further investigation is needed to determine the cause of this difference and possibly in the design of the vaccine.