Background
A number of seaweed species are consumed as food in several countries and documented as drugs in traditional Chinese medicine. Fucoidan extracted from L. japonica is an antioxidant, with the fatty acid composition of n–3 fatty acids, polysaccharides, vitamins, minerals and trace elements (Jeong et al., 1993), and minor compounds such as sterols. Saccharina japonica is also well known for several biological activities, including antioxidant, anti-mutagenic, and antibacterial activities (Okai et al., 1993; Wang et al., 2006; Park et al., 2009).
Recently, many studies have reported on prospective natural resources regulating the serum cholesterol and triglyceride (TG) levels (Ghule et al., 2006; Lemhadri et al., 2006). Hypercholesterolemia and hyperlipidemia are important risk factors in the initiation and progression of atherosclerotic disease (Goldstein et al., 1973; Harrison et al., 2003). Hypercholesterolemia is characterized by an increase in serum lipids such as TC, low-density lipoprotein cholesterol (LDL-C), and TG (Levine et al., 1995). Hyperlipidemia mainly demonstrates increased levels of total cholesterol (TC), TG, and LDL-C, along with a decrease in the high-density lipoprotein-cholesterol (HDL-C). Studies have indicated the potential of synthetic and natural sources that could regulate plasma TC and TG levels in coronary atherosclerosis (Ghule et al., 2009).
The sea tangle is often used as a functional food or alginate extraction material in Korea and Japan. The alginate-free residue of sea tangle is discarded as waste. For the purpose of high value-added use of the alginate-free residue of sea tangle, we investigated the anti-hyperlipidemic and anti-atherosclerotic effects of the alginate-free residue from sea tangle.
Methods
The sea tangle (Saccharina japonica) was obtained from a local supplier (Gangneung, Gangwon-do, Korea) in March 2007. Poloxamer-407 (Pluronic F-127) and corn oil were purchased from Sigma (St. Louis, MO, USA). TC (Cholestezyme-V), TG (Triglyzyme-V), and high-density lipoprotein-cholesterol (HDL-C; HDL-C555) were assayed using commercially available kits (Asan Pharm. Co., Ltd., Korea).
The functional compounds (M1 and M2) in the alginate-free residue of sea tangle were effectively extracted by supercritical fluid extraction (SFX 3560, Lincoln, USA). Supercritical CO2 was used as a solvent and extraction was performed using 1.0 g sea tangle in a 10-mL extractor. The extraction was performed for 20 min with a fluid flow rate of 1.0 mL/min, measured at the pump head. The extraction was performed at 40 °C and 6500 psi in the sample cartridge for 10 min, followed by extraction through the cartridge at 70 °C. The extracted sample was collected in collection vial with ethanol.
Sprague-Dawley male rats weighing 130–150 g were obtained from the Dae-han Biolink Co., Ltd. (Chungbuk, Republic of Korea), maintained under constant conditions (temperature 20 ± 2 °C, humidity 40–60%, light 12-h cycle) and acclimatized for 1 week. The rats had free access to drinking water, with the feed prepared according to the recommendations of the American Institute of Nutrition (AIN-76). After the animals were fed the AIN-76 diets, 50 or 100 mg (lipid solution/kg of body weight in 5% Tween 80) of the alginate-free residue extracted from sea tangle powder was orally administered, once a day for 2 weeks. Following this period, the rats were fasted for 24 h and killed and dissected under CO2 anesthesia. All animal experiments were approved by the University of Kyungsung Animal Care and Use Committee.
The poloxamer-407 hyperlipidemic diet model was determined according to the method described by Wout et al. (1992). The rats were administered a 300 mg/kg dose of poloxamer 407 intraperitoneally, prepared by combining the agent with saline solution.
The Triton WR-1339 hyperlipidemic diet model was performed according to the method described by Kusama et al. (1998). Triton WR-1339 (200 mg/kg) was injected into the tail vein after a fasting period of 16 h. After inducing hyperlipidemia, the animals were anesthetized with the CO2 gas and blood was gathered for analysis 18 h later.
According to Duhault et al. (1976), we administered corn oil in the diet at 3 g/kg. The compositions of the normal and high-fat diet are shown in Table 1. The high-fat-diet–treated rats were orally administered the test substance for the last week, with the high-fat diets fed daily for 6 weeks.
AIN76 mixture: Nutritional Biochemicals, ICN Life Science Group, Cleveland, Ohio
Based on the method of Folch et al. (1957), the lipids were extracted by homogenization of the feces with 2:1 chloroform-methanol (v/v), followed by centrifugation. The lipids were extracted based on the dry weight of the feces and assayed for TC and TG concentration using a standard enzymatic assay kit (Asan Pharm., Korea).
The levels of TG, TC, and HDL-C were determined by enzymatic colorimetric methods using commercial kits (Shinyang Chemical Co., Busan, Korea). The concentration of LDL-C was calculated using the following equation (Friedwald et al., 1972).
LDL-C = TC–HDL-C–(TG/5)
High-performance liquid chromatograph (HPLC, Hitachi, Tokyo, Japan) system was performed using a Lichrospher RP-18e column (8 × 250 mm, Merck). The mobile phase used was methanol/acetonitrile (7:3, v/v) at a flow rate of 1.0 mL/min, and detection was performed at 450 nm and 210 nm by a diode array detector (L7455 type, Hitachi). The amounts of M1 fractions were quantified from their peak area by the use of a standard curve identified with fucosterol.
All results are presented as the mean ± SD. Data were evaluated by one-way ANOVA using SPSS (IBM SPSS, Armonk, NY, USA), after which the differences between the means values were assessed using Duncan’s multiple range test. Results were considered statistically significant at P < 0.05.
Results
We assessed the effect of the oral administration of M1 and M2 100 mg/kg of body weight, once a day for 2 weeks, on the serum lipid levels in poloxamer 407-induced hyperlipidemic rats. Serum TG and TC levels were reduced by M1 and M2 when compared to the control rats, in poloxamer 407-induced hyperlipidemic rats (Table 2). M1 demonstrated a more potent effect on the serum lipid levels than the M2 fraction. Hence, we proceeded to assess if M1 possessed a dose-dependent effect. The administration of the M1 at a dose of 50 and 100 mg/kg body weight significantly reduced serum lipid levels when compared to the control rats (Table 3).
Values represent mean ± S.D. (n = 8). Values sharing the same column superscript letter are not significantly different from each other (P < 0.05) by Duncan’s multiple range test
Values represent mean ± S.D. (n=9). Values sharing the same column superscript letter are not significantly different from each other (P < 0.05) by Duncan’s multiple range test
Rats with hyperlipidemia induced by Triton WR 1339 demonstrated remarkably high serum levels of TG, TC, and LDL-C. However, the administration of the M1 at doses of 50 and 100 mg/kg body weight significantly reduced the TG levels in the hyperlipidemic rats as compared to the control rats (Table 4). The TC and LDL-C were reduced in the M1-treated groups compared with control rats; however, no dose-dependent differences were observed between M1 and M2.
Values represent mean ± S.D. (n=9). Values sharing the same column superscript letter are not significantly different from each other (P < 0.05) by Duncan’s multiple range test
Table 5 shows the serum lipid levels following the oral administration of M1 50 and 100 mg/kg body weight. The serum lipid levels such as TG and TC were remarkably increased in the control rats induced corn oil; however, the administration of M1 significantly reduced the serum TG and TC levels.
|
Treatment |
Dose |
Triglyceride |
Total cholesterol |
|---|---|---|---|
|
mg/kg |
mg/dL |
||
|
Normal |
86.8 ± 10.5c |
73.5 ± 8.56c |
|
|
Control |
230.7 ± 19.7a |
98.8 ± 7.79a |
|
|
M1 |
50 |
197.6 ± 20.3b |
91.6 ± 9.66a |
|
100 |
172.9 ± 11.1b |
86.7 ± 5.24b |
|
Values represent mean ± S.D. (n=9). Values sharing the same column superscript letter are not significantly different from each other (P < 0.05) by Duncan’s multiple range test
The effects of M1 on the serum lipid levels of rats fed a high-fat diet are shown in Table 6. The rats fed a high-fat diet reported significantly increased levels of serum of TG, TC, and LDL-C compared to the normal rats. The serum lipid levels including TG, TC, and LDL-C were significantly reduced by M1 100 mg/kg, with no reduction observed in the serum lipid levels of the control rats (Table 6). The abdominal fat pad weights in the normal and diet-induced obesity rats fed with M1 were also assessed. The weights of the retroperitoneal WAT, epididymal WAT, and total abdominal WAT per body weight of rats were significantly lower in the diet-induced obesity rats treated with M1 100 mg/kg body weight than the control rats (Table 7). The fecal contents of the diet-induced obesity rats were not altered when compared to dose-dependent of M1. The rats fed the M1 100 mg/kg of body weight diet decreased of total lipid, TG and TC (Table 8). The rats fed M1 100 mg/kg reported lower blood leptin and insulin levels than the control rats (Table 9).
Values represent mean ± S.D. (n=9). Values sharing the same column superscript letter are not significantly different from each other (P < 0.05) by Duncan’s multiple range test
Values represent mean ± S.D. (n=9). Values sharing the same column superscript letter are not significantly different from each other (P < 0.05) by Duncan’s multiple range test
Values represent mean ± S.D. (n=9). Values sharing the same column superscript letter are not significantly different from each other (P < 0.05) by Duncan’s multiple range test
|
Treatment |
Dose |
Leptin |
Insulin |
|---|---|---|---|
|
mg/kg |
mg/dL |
||
|
Normal |
8.76 ± 0.47c |
3.56 ± 0.19b |
|
|
Control |
26.25 ± 6.43a |
4.16 ± 0.30a |
|
|
M1 |
50 |
24.83 ± 3.29a |
4.06 ± 0.28a |
|
100 |
16.55 ± 3.10b |
3.97 ± 0.21a,b |
|
Values represent mean ± S.D. (n=9). Values sharing the same column superscript letter are not significantly different from each other (P < 0.05) by Duncan’s multiple range test
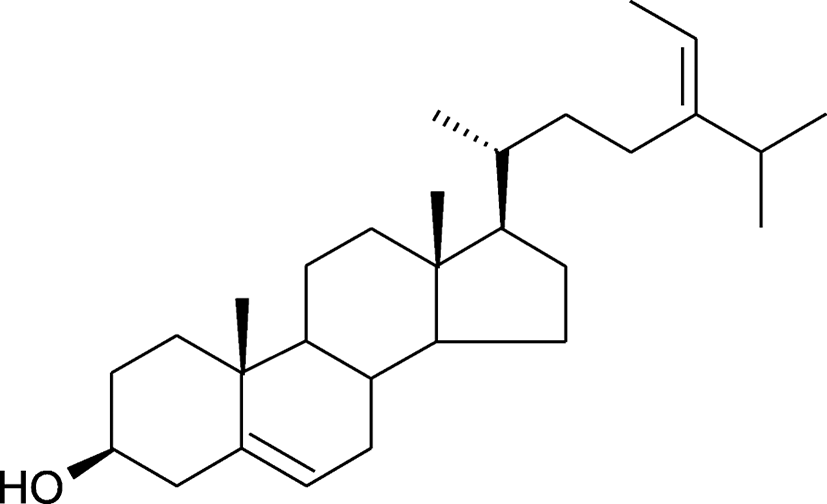
To find a key functional anti-hyperlipidemic compound in M1, the properties were compared with the reference substance after separation using the HPLC (data not shown). The results demonstrated fucosterol as the key functional compound (Fig. 1).
Discussion
Hyperlipidemia, obesity, and diabetes mellitus are chronic diseases associated with serious complications that may consequently increase the risk of atherosclerosis. Thus, regulating the serum cholesterol levels is important, as increased serum levels of TC and LDL-C are the significant determinants in the development of atherosclerosis (Jeong et al., 2010).
In the present study, we investigated the effects of the alginate residue extracted from sea tangle on the serum lipid profile of hyperlipidemic and diet-induced obesity rats. The results demonstrated that M1 administration in the hyperlipidemic rats significantly decreased the serum TC, TG, and LDL-C levels. Previous studies have reported the hypolipidemic effects of edible seaweeds, dietary fiber, plant sterols, and herbal extracts, as indicated by decreased serum TC, TG, and LDL-C levels on rats (Nigon et al., 2001; Ara et al., 2002; Yamada et al., 2003; Megalli et al., 2005; Jeong et al., 2010). According to these studies, lowering serum TC and LDL-C levels plays an important role in reducing the risk of developing atherosclerosis.
In addition, the diet-induced obesity rats treated with M1 reported decreased abdominal fat weight compared to the control rats. These results suggest that the M1 fraction effects obesity by reducing the abdominal fat weight in obese rats. We also investigated the total lipid, TG, and TC levels in the fecal contents of the control and diet-induced obesity rats fed with M1. The M1-treated rats reported increased fecal content of the total lipid, TG, and TC levels. This data indicated that M1 lowered the serum lipid through the increased excretion of total lipid, TG, and TC from the body. Hence, it was concluded that M1 demonstrated hypolipidemic activity in rats. Moreover, lowering the serum cholesterol level is crucial for the prevention of cardiovascular diseases (Hideomi et al., 2005). M1 treatments also exerted anti-hyperlipidemic effects by regulating the serum lipid levels in rats with induced hyperlipidemia. HPLC was performed to confirm the presence of functional components in the M1 fraction, and the identification of fucosterol in the M1 fraction was confirmed by the comparison of retention times with the reference standard. In previous studies, fucosterol, isolated from marine algae Pelvetia siliquosa, has been investigated for anti-oxidant and anti-diabetic activities (Lee et al., 2003; Lee et al., 2004). Furthermore, many studies have reported that among the serum lipids, LDL-C is the most dangerous, as the oxidation of LDL leads to its increased infiltration in the arterial walls (Aviram, 1993). Therefore, reducing the oxidation of LDL-C is essential due to the presumed involvement in the development of atherosclerotic disease.
Conclusions
Our results demonstrated that the alginate-free residue of sea tangle reduces serum levels of TC, TG, and LDL-C. These results suggest that the alginate-free residue of sea tangle contains physiologically active components, such as fucosterol, that may exert beneficial effects in the prevention of atherosclerosis.