Background
The overuse of antibiotics in the environment may have important economic and sanitary outcomes (Martinez 2009; Hatosy and Martiny 2015). Indeed, the release of antibiotics in natural environments exerts a strong pressure on bacteria strains and supports the selection of resistant bacteria. The recurrent use of antibiotics decreases their effectiveness over time (Blair et al. 2015). To reduce the overuse of antibiotics and minimize the impacts to the environment and human society, there is an urgent need for alternatives to antibiotics (Editorials 2013; Spellberg and Gilbert 2014).
In aquaculture antibiotic resistance causes mass mortality of cultured species (Karunasagar et al. 1994) which result in economic loss for farmers (Shrestha et al. 2018). Aquaculture itself largely contributes to the dissemination of antibiotics resistance genes in the aquatic environment (WHO, 2006; Shah et al. 2014), which increases the risks on human health (Aly and Albutti 2014). Policy on antibiotics in aquaculture is becoming more strict, and antibiotics are forbidden in some countries (Lulijwa et al. 2019). Finding antibiotic alternatives in this field is the focus of the current research (Pérez-Sánchez et al. 2018) due to the promising market they represent. To reduce the selective pressure exerted on bacteria strains, novel strategies target natural products that inhibit the expression of virulence genes without exerting a strong bactericide activity (Moloney 2016; Spellberg and Gilbert 2014). Such promising products include inhibitors of quorum sensing (Chen et al. 2018; Pérez-Sánchez et al. 2018) which exhibited in vitro and in vivo effectiveness in aquaculture (Manefield et al. 2000; Brackman et al. 2008; Pande et al. 2013).
Quorum sensing (QS) is a cell-to-cell communication process in bacteria based on the secretion and detection of signal molecules (i.e., autoinducers) by bacteria. Specifically for gram-negative bacteria, autoinducers (AIs) consist of small molecules, mainly acyl-homoserine lactone (AHL) derivatives (Waters and Bassler 2005). Quorum sensing allows the expression of target genes involved in biofilm formation, toxin secretion, and bioluminescence (Henke and Bassler 2004a). It is influenced by the concentration in AIs related to the bacterial density and the genetic similarity of bacteria neighbors (Schluter et al. 2016).
A model species for testing the relevance of antibiotic alternatives in aquaculture is V. harveyi. Vibrio harveyi is a luminescent bacteria inhabiting the marine environment and pathogens in aquaculture, specifically when it is associated with Tenacibaculum maritimum (Reverter et al.2016). The QS of V. harveyi is well documented, with three parallel QS systems that are regulated by three couples of signal molecules and cognate sensors: V. harveyi autoinducer-1 (HAI-1) and LuxN sensor; autoinducer-2 (AI-2) and LuxPQ sensor; Cholerae autoinducer-1 (CAI-1); and CqsS sensor (Henke and Bassler 2004a). Together these three systems encode bioluminescence and virulence factors as biofilm formation, type III secretion, and a secreted metalloprotease genes (Henke and Bassler 2004a; Henke and Bassler 2004b).
Quorum sensing inhibitors (QSIs) of V. harveyi have already been identified from a variety of marine organisms, including bacteria, algae, and sponges (Givskov et al. 1996; Peters et al. 2003; Rasch et al. 2004; Teasdale et al. 2009; Dobretsov et al. 2011; Natrah et al. 2011; Kalia 2013; Tello et al. 2013; Saurav et al. 2017). Marine sponges are promising sources of antibiotic alternatives because (i) they are known to be a reservoir of diverse microbial communities (Thomas et al. 2016) and (ii) as primitive sessile organisms featured with a simple multicellular structure, their main defense against pathogen rely on the production of secondary metabolites with antibiotic and antibiofilm (Feng et al. 2013), and QS inhibition activities against pathogens (Blunt et al. 2005; Müller et al. 2013; Quévrain et al. 2014). In this study, Vibrio harveyi and T. maritimum were used as model species to test antibiotic and QS inhibition bioactivities of two compounds isolated from the sponge Fascaplysinopsis cf reticulata collected in French Polynesia. Because of the cytotoxicity of fascaplysin (2) reported in the literature (Hamilton 2014), we also evaluated the toxicity of F. cf reticulata extract on two fish species (Poecilia reticulata and Acanthurus triostegus) to check the safety of using this sponge in fish farming.
Methods
Sponge samples were collected manually using SCUBA, between 45 and 65 m depth in the Tuamotu Archipelago (French Polynesia) during the 2011 Tuam expedition aboard the Alis vessel (Debitus 2011), on the outer reef of Anuanuaro Atoll (20°25.394’S, 143°32.930’W). Samples were frozen immediately at –20 °C on board until being processed.
The sponge collected was freeze dried and grounded to obtain 95 g of dry sponge powder. It was extracted using 100 ml of 80% ethanol and then rinsed twice in 100% ethanol. The solvent was evaporated under reduced pressure, and the remaining ethanolic extract was dissolved in water and successively partitioned three times with cyclohexane and three times with dichloromethane. The cyclohexanic fraction was subjected to silica gel chromatography (40–60-μm mesh) and then eluted with cyclohexane and ethyl acetate mixtures of increasing polarity. Further semi-preparative HPLC on normal phase column eluted with cyclohexane/ethyl acetate 55/45 vol/vol allowed the isolation of palauolide (1) (5 mg). The purification of the dichloromethane fraction (called fascaplysin-enriched fraction (FEF)) using reverse phase HPLC (column: Interchrom Uptisphere strategy, 5 μm; solvent: (water/acetonitrile 70:30), TFA 0.1%) led to the isolation of fascaplysin (2) (17% of FEF, 0.02% dry sponge weight, 19 mg). High-performance liquid chromatography analysis was performed on HPLC (Agilent Technologies 1260 Infinity) with diode array (Agilent G1315C) and evaporative light-scattering (Agilent G4260C) detection. Yields were calculated using the ratio compound weight/freeze-dried sponge weight. Structure elucidation of the two known compounds was performed on the basis of 1H and 13C NMR and mass spectra.
The toxicity effect of F. cf reticulata’s FEF on fish was evaluated on two fish species that can be easily found in French Polynesia and reared in the laboratory: P. reticulata (the guppy or mosquito fish) and A. triostegus (the convict tang fish). Poecilia reticulata specimens (5–8 cm length) were collected from a freshwater pool at Tahiti at night. Poecilia reticulata specimens were appealed with a flashlight and then caught with a landing net (5-mm mesh size) and kept in 3 L plastic jar containing freshwater. Young settlers (or recruits, 1,5, 2 cm length) and juveniles (3–7 cm length) of A. triostegus (at the two distinct developmental stages) were caught during full moon nights on the foreshore puddles and on the reef crest using a net of the northeast coast of Moorea Island (17°29'52.19"S, 149°45'13.55”W). Acanthurus triostegus recruits (fish larvae undergoing metamorphosis) were transparent at the time of capture, demonstrating that they had just entered the reef following their pelagic larval stage, while the juveniles (old settlers, already metamorphosed and settled when captured) were already fully pigmented when caught, demonstrating that they had already settled in this reef area for at least a week (Lecchini et al. 2004).
A preliminary assay was performed on P. reticulata by balneation, as described previously for environmental toxicity studies of acetylcholinesterase (AChE) inhibitor pesticides (Wester and Vos 1994; Bocquené and Galgani 2004; El-Demerdash et al. 2018). Fascaplysin-enriched fraction ethanolic solution was further tested in duplicate at 1 and 5 μg ml–1 during 72 h (chronic toxicity) and at 50 μg ml–1 during 1 h (acute toxicity) in 2-L tanks, each containing five fishes. Solvent controls were run for each experiment. For the 72-h experiment, water, FEF, and EtOH were renewed, and fishes were fed once a day with commercial flakes. Abnormal behavior of fishes after exposure to FEF was evaluated qualitatively, such as swimming difficulties (i.e., irregularity of swim velocity, asymmetric pectorals fins movements, upside down swimming, and quick jumps) and loss of appetite.
Since the preliminary assay highlighted a modification of P. reticulata behavior by FEF (see results section), a second toxicity assay was performed on A. triostegus focusing on feeding behavior by using a quantitative method. The effect on FEF exposition on A. triostegus feeding behavior was assessed on two distinct developmental stages in order to compare the activity of FEF at both stages of development. The bioassays on A. triostegus were performed in 3-L tanks. Fishes (young settlers or juveniles) were exposed to FEF at 1 μg ml–1 in groups of four or five individuals during 24, 48, and 72 h. Rubble with encrusting turf algae were placed in the tank for fish to feed on 1 h per day during 3 days. The feeding behavior was assessed by counting the number of bites on the algae encrusted rubble in each aquarium. Six video sequences of 5 or 10 min per aquarium per day were analyzed. Results are expressed in number of bites per fish per hour.
Every purified compound was tested in triplicate at four concentrations, 1, 5, 10, and 50 μg ml–1 against the wild strain V. harveyi BB120 (Johnson and Shunk 1936; Bassler et al. 1997), and three derived mutants, JAF 375 (Freeman and Bassler 1999), JMH 597, and JMH 612 (Henke and Bassler 2004a). All strains were obtained from Bassler laboratory (Bassler et al.1997; Freeman and Bassler 1999; Henke and Bassler 2004a). Each mutant only expressed one of the three QS systems of V. harveyi: JAF 375 (CAI-1 activated), JMH 597 (AI-2 activated), or JMH 612 (HAI-1 activated) (Freeman and Bassler 1999; Henke and Bassler 2004a). Quorum sensing inhibition bioassay was performed by combining simultaneously luminescence kinetics (in relative luminescence units, RLU) and absorbance kinetics (at λ = 600 nm) (Givskov et al. 1996; Brackman et al. 2008; Steenackers et al. 2010). Absorbance kinetics was used to measure the growth of V. harveyi with any tested compound or controls. Data was obtained using a Fluostar Omega spectrophoto-luminometer (BMG Labtech Fluostar OPTIMA, Ortenberg, Germany).
The quorum sensing inhibition bioassay was modified from Mai et al. (2015). A V. harveyi colony was grown on Zobell agar plates (BD Bacto™ peptone, 5 g; BD BBL™ yeast extract, 1 g; BD Bacto™ agar, 17 g; sterilized sea water, 1 L) for 24 h. The plates were then suspended in liquid Lennox L broth base medium (Invitrogen, Carlsbad, CA, USA) which was supplemented with artificial sea salts (Sigma Aldrich Co., St Louis, MO, USA) at 40 g l–1 and was then incubated for 16 h under constant orbital stirring at 27 °C. This suspension (50 μl) was then diluted in Marine Broth (CONDA®, Madrid, Spain) (10 ml) and was incubated for 30 min while stirring at 27 °C. Compounds were dissolved in absolute ethanol, deposited in sterile 96-μClear® bottom wells microplates (Greiner Bio-One, Germany) that were dried at room temperature under a laminar flow hood. Each sample was tested in triplicate for each concentration of purified compound tested (1, 5, 10, and 50 μg ml–1). Compounds were then dissolved in Marine Broth (100 μl) by sonication at 50/60 Hz for 30 min, and a bacterial suspension (100 μl) was added in the appropriate wells. The 96 wells plates were incubated at 27 °C for 12 h in a microplate incubator reader, with luminescence and absorbance reading conducted every 10 min, after 1 min of double orbital stirring. The sterility of the culture medium was checked throughout the experiment, as well as the absorbance of each tested compound. Luminescence and absorbance data at the N-cycle reading (L N-cycle and A N-cycle) were respectively obtained after subtracting the mean of the first ten cycles of the luminescence and absorbance (L mean first 10 cycles and A mean first 10 cycles) from the raw data (L N-cycle raw data and A N-cycle raw data) (Eqs. 1 and 2).
The kinetic curves obtained were sigmoidal. Any delay or inhibition of both growth and luminescence curves compared to the control curves (which mean an inhibition of growth rate) is translated to an antibiotic effect of the compound. By contrast, no change in bacterial growth between tested and control curves associated with a delay of luminescence between tested and control curves translated to a QSI effect of the compound.
Antibiotic activity on T. maritimum could not be performed through the absorbance kinetics method as previously described for V. harveyi strains, because T. maritimum precipitated at the beginning of the experiment which prevented measuring absorbance. Antibiotic activity on T. maritimum was tested using the disk diffusion method on solid agar medium (Bauer et al. 1966). This bioassay was performed on a strain of the marine bacteria named TFA4 (Reverter et al. 2016). Pure compounds were dissolved in 100% ethanol to obtain impregnated disks (cellulose disks, 6 mm diameter) with 0.5, 0.25, 0.125, and 0.0625 μg of compound. Disks were air-dried in a laminar flow cabinet and then deposited on Zobell agar plates, previously seeded with TFA4 strain. Petri dishes were incubated at 27 °C for 2 days.
Absorbance was modeled as a logistic function of time (t) (Kingsland 1982) according to Equation 3, where Amax is the maximum or asymptotic value of absorbance, k is the steepness of the curve, and t0 is the x value of the sigmoid’s midpoint.
Luminescence was also modeled as a logistic function, following Equation 4, where Lmax is the maximum or asymptotic value of luminescence. Equation 4 includes an a parameter to adequately model the high steepness found for luminescence curves. For each compound and concentration tested, the parameters of the logistic curve were fitted using the function “nls” of the package “stat” in R.3.1.0. The effect of compounds on the growth and the bioluminescence of V. harveyi populations were evaluated by comparison of the growth rate (assimilated to the parameter k) and the curve inflection points. For the absorbance kinetics, the inflection point was equal to t0. For the luminescence kinetics, the derivative (Y’) of the sigmoid function was calculated, and the inflection point was identified as the time for which Y’ was maximal. Furthermore, to provide comparable values of bioluminescence, luminescence values were compared at a fixed bacterial concentration (i.e., fixed absorbance A = 0.055, which corresponded to half the maximum absorbance Amax of control).
For all parameters involved in QS activity (k and inflection points) as well as in toxicity (number of bites per unit of time per fish), differences between concentration were tested using the non-parametric Kruskal-Wallis test (function kruskal.test of pgirmess package in R.3.1.0) and a multiple comparison test after Kruskal-Wallis (function kruskalmc), suitable for small samples. A QSI activity was evidenced when (1) V. harveyi population growth rate (k, see Eq. 3) was not significantly lower with compound (or extract) compared to control (Kruskal-Wallis test and multiple comparison test after Kruskal-Wallis, α = 0.05) and (2) the inflection point of luminescence is significantly higher with compound (or extract) compared to control (Kruskal-Wallis test and multiple comparison test after Kruskal-Wallis, α = 0.05).
Results
The hydro-alcoholic extraction of sponge powder (95 g) provided 2.8 g of extract. The partitioning of this extract led to cyclohexanic (1.46 g, yield 1.54% w/w) and dichloromethane (0.112 g, yield 0.11% w/w) fractions. The purification of the cyclohexanic fraction conducted to the known palauolide (1) (0.005 g, yield 0.005% w/w) and the dichloromethylenic fraction to the alkaloid fascaplysin (2) (0.019 g, yield 0.02% w/w) (Fig. 1).
Absorbance and luminescence kinetics of the V. harveyi wild strain (Fig. 2 a and b) highlighted a dose-dependent effect of palauolide (1) on BB120 bacterial growth. During the growth of V. harveyi bacterial strains, the growth rate (k parameter) of absorbance increased as the concentration of palauolide (1) increased (Table 1, Fig. 2c). As a consequence, the sigmoid midpoint (t0) decreased as concentration of palauolide (1) increased (data not shown). At 50 μg ml–1 of palauolide (1), the growth rate of absorbance (k = 0.0127 ± 0.0005) reached values significantly higher than for controls (k = 0.0086 ± 0.0008; multiple comparison test after Kruskal-Wallis; p < 0.05). Also not significant due to the lack of statistical power, similar trends were obtained for the three derived QS mutants (Table 1). Despite the stimulating effect of palauolide (1) on V. harveyi growth, a delay in luminescence activation of approximately 17 min was observed for the highest concentrations tested 50 μg ml–1, compared to the luminescence curve of the control (Fig. 2b, red and black curves, respectively). At the same growth stage (A = 0.055), a decrease in RLU was observed for the highest concentration of palauolide (1) compared to control. Such decrease was found for the BB120 wild strain (RLU respectively at 106 210 ± 24 385 at 50 μg ml–1 (26 μM) of palauolide (1) compared to 172 416 (± 2 489) for control; Table 1; Fig. 2d) and the JMH 612 mutant only (RLU respectively at 99 806 ± 18 002 at 50 μg ml–1 (26 μM) of palauolide (1) compared to 189 392 ± 2 609 for control; Table 1; Fig. 2d). For the JMH 612 mutant, the delay between the luminescence kinetics at 50 μg ml–1 and the luminescence kinetics of control was 50 min in average. These results indicate that palauolide (1) boosted bacterial growth and inhibited V. harveyi QS through HAI-1 QS pathway.
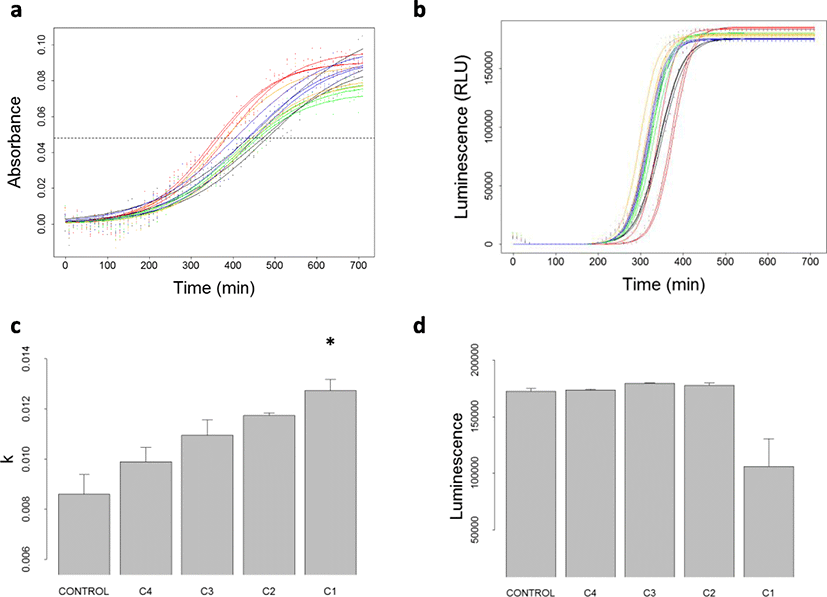
Control, C4, C3, C2, and C1, respectively, refer to palauolide (1) concentration at 0, 1, 5, 10, and 50 μg ml–1. Estimated values of k and RLU are provided for each replicate (n = 3) and for all replicates (mean ± standard deviation over the three replicated). Values significantly different from controls (α = 0.05) are marked with a star (*)
Vibrio harveyi BB120 population growth rate (k, see Eq. 3) was significantly lower with fascaplysin (2) at 50 μg ml–1 (k = 0.0021) compared to control (k = 0.0121; p value < 0.05). Similar results were obtained for mutant JAF 375, with lower growth rate (k = 0.0036) and with fascaplysin (2) at 50 μg ml–1 compared to control (k = 0.0119). Strong decreases of population growth rate were also obtained for mutants JMH 597 and JMH 612 with fascaplysin (2) at 50 μg ml–1 compared to control. For several replicates involving the two last mutants, population growth was null or negative with fascaplysin (2) at 50 μg ml–1, which prevented the growth model to be fitted and k estimates to be provided (Table 2; Additional file 1). This suggests an antibiotic effect of fascaplysin (2) on V. harveyi and prevents concluding on a QS inhibition effect.
Palauolide did not display any antibiotic activity against the marine pathogen T. maritimum. By contrast fascaplysin (2) displayed antibiotic activity at 0.25 μg per disk (11 mm) and 0.5 μg per disk (18 mm) against T. maritimum (TFA4) (disk diffusion bioassay).
At 50 μg ml–1 of FEF, P. reticulata exhibited signs of hyperventilation as well as motility disarrangement (i.e., jerky movements with sudden accelerations or motionless periods) within the first hour of treatment. None motility disarrangement was observed at 1 μg ml–1 FEF solutions, but changes to the feeding behavior were noticed for P. reticulata, i.e., P. reticulata tasted the food flakes but did not ingest them. At 5 μg ml–1 of FEF, all of the P. reticulata died within 12 h.
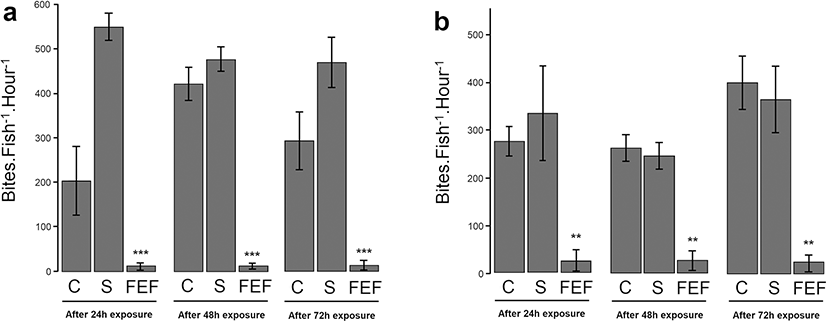
The experiment on A. triostegus was only performed at 1 μg ml–1 of FEF. For each time of incubation (24, 48, and 72 h), the number of bites of A. triostegus (both recruits and juveniles) decreased significantly compared to control A. triostegus (Fig 3). After 24 h of incubation with 1 μg ml–1 FEF solution, the number of bites decreased by 91.3% (± 1. 6%, p value < 0.01) for recruits and by 95.9% (±0.8%, p value < 0.001) for juveniles compared to the control A. triostegus (Fig 3). This trend was confirmed for others times of exposition.
Discussion
The isolation of palauolide (1) and the major compound fascaplysin (2) from the French Polynesian F. cf reticulata extracts is similar to those results obtained by Sullivan and Faulkner (1982) on Palauan sponges.
The QSI potential of the French Polynesian sponge F. cf reticulata against the QS-dependent phenotypic expression in V. harveyi was demonstrated for the first time. Palauolide (1) revealed a potential as QSI by inhibiting V. harveyi luminescence at 26 μM. In quantitative analysis, palauolide (1) delayed the activation of bioluminescence expression to 50 min of V. harveyi BB120. The V. harveyi growth rate was also significantly increased (p value < 0.05). The boosted growth rate of V. harveyi with palauolide (1) can be interpreted as a consequence of QS inhibition, because the expression of bioluminescence slows down bacterial growth rate to save energy (Nackerdien et al. 2008). The present data corroborates well with the result obtained previously on the QSI at 23 μM of isonaamidine A isolated from the sponge Leucetta chagosensis (Mai et al. 2015). Other studies compared bioluminescence data at a time t, to determine the inhibition of QS (Brackman et al. 2008; Teasdale et al. 2009; Natrah et al. 2011). For example, Brackman et al. (2008) showed inhibition of V. harveyi bioluminescence with cinnamaldehyde and derivatives at 100 μM, 6 h after the addition of compounds (Brackman et al. 2008). Skindersoe et al. (2008) found that manoalide, a compound of similar structure to palauolide (1), inhibits QS at IC50 = 0.66 μM. The better bioactivity of manoalide compared to palauolide (1) could be explained from the sensitivity of the intracellular bioassay used by authors.
The mode of action of palauolide (1) on the inhibition of QS has potential as an antibiotic alternative in aquaculture for Vibrio species. Our bioassay on V. harveyi double mutants JAF 375, JMH 597, and JMH 612 highlighted interference of palauolide (1) on V. harveyi QS, specifically with the acyl-homoserine lactone: HAI-1. Quorum sensing regulates bioluminescence and virulence factors of bacteria through autoinducers (Henke and Bassler 2004a) such as HAI-1 used for intraspecies communication (Waters and Bassler 2005; Yang et al.2011). Acyl-homoserine lactone molecules are found in the family Vibrionaceae (Yang et al.2011). Palauolide (1) can therefore interfere with Vibrio species QS through HAI-1 pathway and then be used as an antivirulent against Vibrio species as antagonist of AIs. Most of antagonists of QS sensors are small molecules (Swem et al. 2008; Gamby et al. 2012) with structural similarities to AIs such as brominated furanone derivatives (Givskov et al. 1996; Rasch et al. 2004; Steenackers et al. 2010). Palauolide (1) is a sesterterpene composed by a δ-hydroxybutenolide moiety and a carbon skeleton. The potential of palauolide as a competitor of HAI-1 is most likely due to its small structure and the moderate polarity of its chemical structure. This enables palauolide (1) to cross over the external membrane lipid of bacteria and to bind on the periplasmic sensors Lux N (Swem et al. 2008). Further research would indicate if there is an antagonist effect of palauolide (1) on the HAI-1 sensor, such as testing against additional V. harveyi mutants (Swem et al. 2008; Blair and Doucette 2013).
Fascaplysin (2) supplies a broad range of biological activity within F. cf reticulata. First, as other β-carboline alkaloids as dysideanin (20 μg) and didemnolines A-D (100 μg), fascaplysin is a strong antibiotic (0.25 μg) (Charan et al. 2002; Hamilton 2014). In the sponge, fascaplysin (2) is the major compound which represents 0.02% of the lyophilized sponge weigh. It exhibits many biological activities including cytotoxicity against tumoral cells (Segraves et al. 2004; Shafiq et al. 2012; Hamilton 2014; Cells et al. 2015; Kumar et al. 2015), antimicrobial activities (Roll et al. 1988), and the inhibition of acetylcholinesterase (Bharate et al. 2012; Manda et al. 2016). For microbial disease treatments in aquaculture, fascaplysin (2) is not ideal. Despite its antibiotic activity against marine pathogens V. harveyi (Table 2) and T. maritimum, fascaplysin (2) is toxic toward both fresh and saltwater fish, P. reticulata and A. triostegus, respectively. Indeed, fascaplysin (2) modified fish behavior and displayed an anorexic effect. The AchE inhibition properties of fascaplysin (Bharate et al. 2012) could explain both its toxicity (Bocquené and Galgani 2004; Modesto and Martinez 2010; Assis et al. 2012) and its effect on loss of appetite of fish (Schneider 2000).
The toxicity of palauolide (1) on fish was not tested in this study because previous work highlighted a weaker cytotoxic activity of palauolide (1) compared to fascaplysin (2) (Charan et al. 2002; Hamilton 2014). However, we recommend performing additional toxicity bioassays of palauolide (1) on fish before using it as alternative of antibiotic in fish farming.
Conclusion
In conclusion, the presence of palauolide (1) and fascaplysin (2) in F. cf reticulata, with QS inhibition and antibiotic properties, respectively, could act as complementary where QSIs help and increase antibiotic action on biofilm formation (Brackman et al. 2011). However, the toxicity on fish of the major compound of F.cf reticulata fascaplysin (2) (yield 0.02% w/w) prevents the use of the sponge extract in fish farming context. We recommend in future research to test toxicity of the cyclohexanic fraction of the sponge and palauolide (1) on fish before concluding on the potential of the cyclohexanic fraction and palauolide (1) as an alternative to antibiotics in fish farming.
