Background
Inflammation is a highly regulated biological process that enables the immune system to efficiently eliminate stimuli and injuries (Masresha et al. 2012). Inflammation can be classified into two subtypes, chronic and acute inflammation, depending on the difference in response time and procedure. Acute inflammation is an initial response of bodies against harmful stimuli. During acute inflammatory response, the plasma and leukocytes are moving from the blood into the injured tissues. There is a shift in the type of mononuclear phagocytic cells in the inflammatory tissues during the course of inflammatory response, from pro-inflammatory to anti-inflammatory or wound healing type.
The inflammatory response is associated with the release of the inflammatory mediators, including nitric oxide (NO), prostaglandins, histamine, and bradykinin from various immune cells such as mononuclear phagocytes and mast cells (González Mosquera et al. 2011; Wijesinghe et al. 2014a). Inflammation is crucial in many diseases such as arthritis, atherosclerosis, cancer, and some deadly diseases (Lee and Weinblatt 2001; Firestein 2006; Klegeris et al. 2007; Wen et al. 2016; Wijesinghe et al. 2013; Wijesinghe et al. 2014b). Therefore, there is increasing attention on finding safe and effective anti-inflammatory agents and elucidating their anti-inflammatory mechanisms.
Algae are considered as a potential bio-resource for the development of functional food, cosmeceutical, and pharmaceutical because they are rich in various bioactive compounds, such as proteins, polysaccharides, phenolic compounds, and sterols (Kim et al. 2016; Sanjeewa et al. 2016; Fernando et al. 2017b). These compounds possess broad-spectrum bioactivities including anti-bacterial, anti-inflammatory, antioxidant, and anti-cancer activities (Jung et al. 2008; Heo et al. 2010; Lee et al. 2012; Lee et al. 2013; Oh et al. 2016; Fernando et al. 2017a).
Spirogyra sp. is freshwater green alga. It has been used as a bio-sorbent to remove heavy metal ions from wastewater (Khalaf 2008). Recently, the pharmacological activities of Spirogyra sp, such as antioxidant, ultraviolet (UV)-protective, and anti-hypertension activities, have been reported (Kang et al. 2015; Lee et al. 2016; Wang et al. 2017). However, the anti-inflammatory activities of Spirogyra sp. have not been evaluated so far. Therefore, in the present study, ethanol extract of Spirogyra sp. (SPE) and the fractions from SPE were prepared, and their anti-inflammatory activities were evaluated in lipopolysaccharides (LPS)-stimulated RAW264.7 cells. The effect of sterol-enriched fraction (SPEH) from SPE on the production of pro-inflammatory mediators was investigated by enzyme-linked immunosorbent assay (ELISA) and western blot analysis. In addition, the in vivo anti-inflammatory effect of SPEH was evaluated using a zebrafish model.
Methods
LPS, acridine orange, 2′,7′-dichlorofluorescin diacetate (DCFH2-DA), dimethyl sulfoxide (DMSO), and diaminofluorescein-FM diacetate (DAF-FM DA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS), penicillin–streptomycin (P/S), and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Gibco/BRL (Grand Island, NY, USA). The ELISA kits for measuring prostaglandin E2 (PGE2), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and IL-1β levels were purchased from R&D Systems Inc. (Minneapolis, MN, USA). Antibodies against β-actin, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Secondary anti-rabbit and anti-mouse IgG antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Other chemicals and regents used in the present study were analytical grade.
Sampling, processing, extraction, and fractionation were performed following the protocols described in the previous study (Wang et al. 2017). In brief, lyophilized Spirogyra sp. powder was extracted by 70% ethanol; the 70% ethanol extract of Spirogyra sp. (SPE) was fractionated by hexane, chloroform, and ethyl acetate, respectively. Then, the hexane (SPEH), chloroform (SPEC), ethyl acetate (SPEE), and water (SPEW) fractions of SPE were obtained. Samples were concentrated and stored at – 20 °C until used.
The sterol contents of samples were measured by Liebermann Burchard method described by Xiong et al. (2007). In brief, the samples (2 mg) were dissolved in glacial acetic acid (1 mL) and mixed with prepared Liebermann Burchard regent. The mixture was reacted in the dark at the room temperature for 10 min. After reaction, the absorbance of the mixture was recorded at 625 nm using a micro-plate reader (BioTek, Synergy, HT, USA). Cholesterol was used as standard sterol compound to prepare the standard curve.
RAW 264.7 cells (ATCC® TIB-71™) were cultured in DMEM containing FBS (10%) and P/S (1%) under a humidified atmosphere containing 5% CO2 at 37 °C. RAW 264.7 cells were seeded as a density of 1 × 105 cells/mL for experiments.
RAW 264.7 cells were seeded and incubated for 24 h. Cells were treated with different concentrations of samples and incubated for 1 h. After incubation, LPS (1 μg/mL) was introduced to cells. The culture medium was collected and used for measuring NO, PGE2, TNF-α, IL-6, and IL-1β production levels. The NO production levels of LPS-treated RAW264.7 cells were measured using Griess reagent. The PGE2, TNF-α, IL-6, and IL-1β production levels were evaluated using the commercial ELISA kits following the manufacturer’s instructions. The cell viability was measured by MTT assay based on the method described by Wang et al. (2018).
The LPS-stimulated RAW 264.7 cells were harvested and lysed. The lysates contain 50 μg of protein were subjected to electrophoresis on SDS–polyacrylamide gels (12%). Then, the proteins were transferred onto nitrocellulose membranes, and the membranes were incubated with the primary antibodies at 4 °C. After 6 h incubation, the membranes were incubated with HRP-conjugated secondary antibody and visualized using ECL reagent (Amersham, Arlington Heights, IL, USA).
Approximately 7–9 h post-fertilization (hpf), zebrafish embryos (15 embryos/group) were treated with different concentrations of SPEH for 1 h. Then, LPS (10 μg/mL) was added into the embryo medium. The survival rate was measured at 3 days post-fertilization (dpf) by counting live embryos, and heart-beating rate was measured following the protocol described by Sanjeewa et al. (2018).
At 3 dpf, zebrafish were dyed with DCFH2-DA (20 μg/mL), acridine orange (7 μg/mL), and DAF-FM-DA solutions (5 μM) for the detection of ROS generation, cell death, and NO production, respectively. The anesthetized zebrafish were photographed under the microscope equipped with Cool SNAP-Procolor digital camera (Olympus, Japan). The fluorescence intensity of individual zebrafish was quantified using an Image J program.
The data were expressed as the mean ± standard error (SE). One-way ANOVA test (using SPSS 11.5 statistical software) was used to compare the mean values of each treatment. The significant differences were established as *p < 0.05, **p < 0.01 as compared to LPS only-treated group and ##p < 0.01 as compared to control group.
Results and discussion
Algae-derived compounds possess various health benefits (Mayer and Hamann 2003; Athukorala and Jeon 2005; Heo et al. 2005; Kotake-Nara et al. 2005). The sterols including cholesterol, β-sitosterol, campesterol, and polyhydroxylated sterols isolated from algae were shown to strong bioactivities, especially anticancer and anti-inflammatory activities (Kazłowska et al. 2013; Elbagory et al. 2015). The freshwater green alga, Spirogyra sp. contains various bioactive compounds. In our previous studies, we have reported antioxidant, UVB photoprotective, and anti-hypertension activities of phenolic compounds isolated from Spirogyra sp. (Kang et al. 2015; Wang et al. 2017). However, the bioactivities of sterols from Spirogyra sp. have not been evaluated yet. In the present study, we have prepared a sterol-enriched fraction (SPEH) from Spirogyra sp. and evaluated its in vitro and in vivo anti-inflammatory activities.
As shown in Fig. 1, the sterol contents of SPE and its fractions were ranged from 0.50% to 9.08%. This result indicates that the sterol content in hexane fraction (SPEH) was enriched with 6.7 times than SPE. Many reports support sterols isolated from algae possess strong anti-inflammation activity (Ku et al. 2013; Sanjeewa et al. 2016; Suh et al. 2018). Our previous study investigated anti-cancer and anti-inflammatory activities of the sterol-rich fraction of Nannochloropsis oculata. The results indicated that the hexane fraction of methanol extract of N. oculata is rich in sterol content and possesses strong anti-inflammatory activity. In this regard, anti-inflammatory activity can be expected from SPEH prepared in this study, in which the content of sterol was 9.08%.
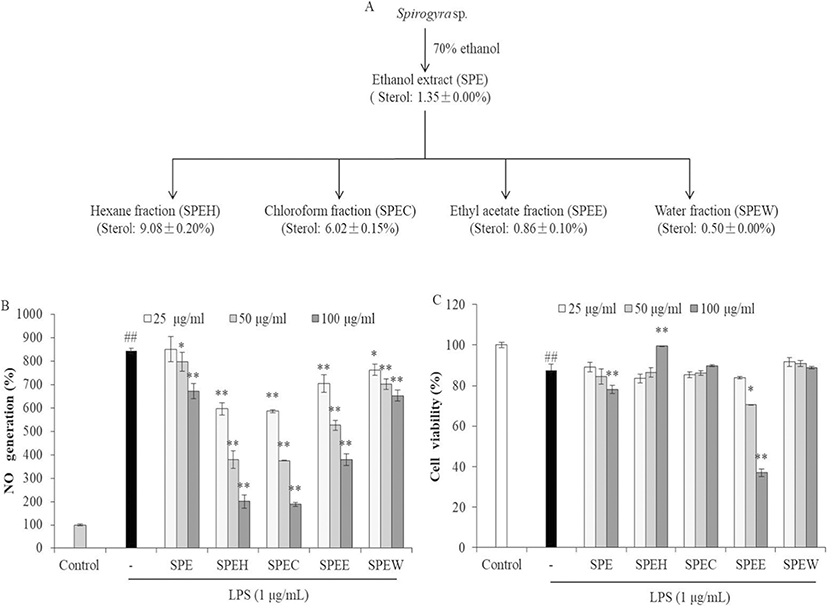
In order to evaluate the anti-inflammatory activity of SPE and its fractions, their inhibitory effects on NO production were measured. As shown in Fig. 1, all samples significantly and dose-dependently decreased NO production (Fig. 1b). However, SPE and SPEE showed remarkable cytotoxicity in RAW264.7 cells. SPEH and SPEC that contain relative higher sterol contents showed stronger NO inhibitory effect in LPS-induced RAW264.7 cells than other samples. Furthermore, SPEH significantly improved the viability of LPS-induced RAW264.7 cells at high concentration. The viability of the cells treated with 100 μg/mL of SPEH was 99.52%, which is close to the cells not treated with LPS (100%) (Fig. 1c). These results indicate that SPEH showed the most potent anti-inflammatory activity among 5 samples. Thus, SPEH was selected as the target sample for the further study.
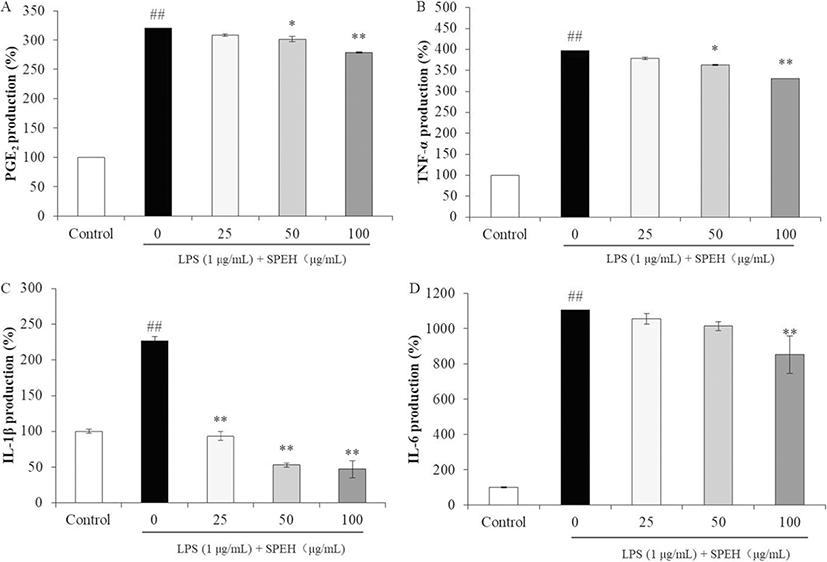
We next examined the inhibitory effects of SPEH on the production of PGE2 and pro-inflammatory cytokines in LPS-stimulated RAW264.7 cells. As Fig. 2a shows, LPS induced PGE2 level in RAW264.7 cells to 319.85% compared to the control group (100%). However, the PGE2 levels of LPS-treated cells were decreased to 307.83%, 301.38%, and 278.90% by 25, 50, and 100 μg/mL of SPEH treatment, respectively. In addition, SPEH inhibited 17.70%, 33.01%, and 65.76% of TNF-α (Fig. 2b), 113.31%, 173.91%, and 179.67% of IL-1β (Fig. 2c), 50.233%, 91.22%, and 252.14% of IL-6 expressions (Fig. 2d) at the concentration of 25, 50, and 100 μg/mL, respectively.
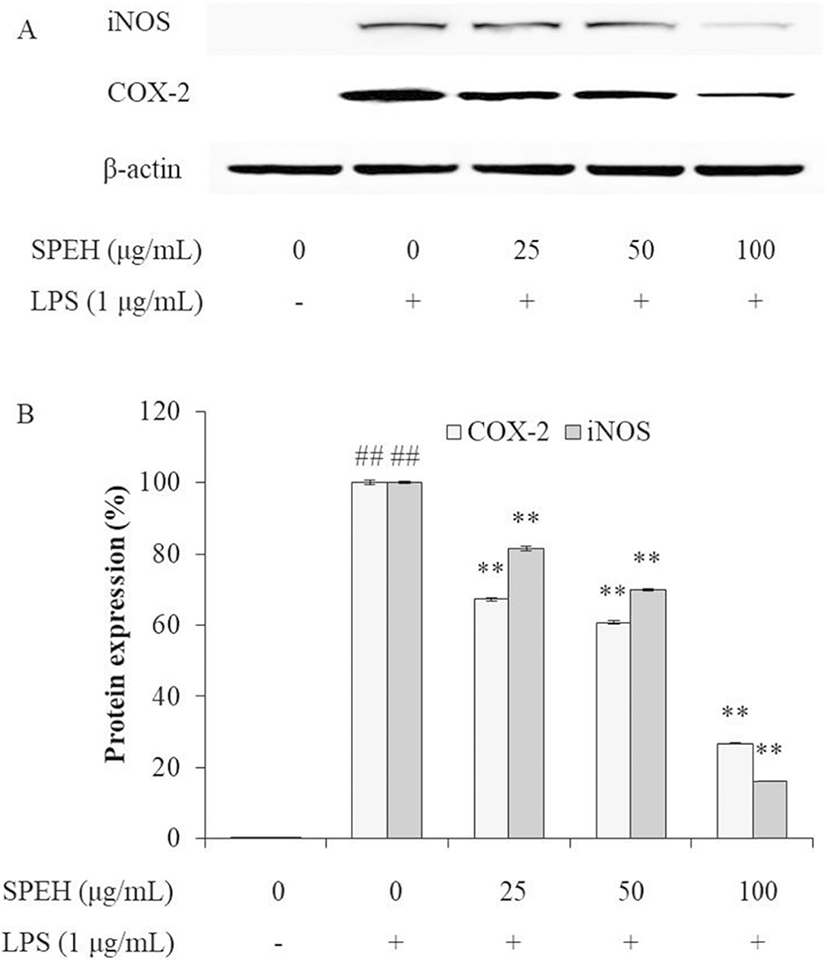
iNOS and COX-2 are two inducible enzymes, which synthesize two key inflammatory mediators, NO and PGE2, respectively (Kim et al. 2005; Wijesinghe et al. 2014a). The levels of iNOS and COX-2 are up-regulated in inflammatory response (Wijesinghe et al. 2014a; Wijesinghe et al. 2014b; Xiong et al. 2014). Therefore, we investigated the effect of SPEH on the protein expression levels of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells. As shown in Fig. 3, LPS significantly stimulated iNOS and COX-2 expressions, but SPEH remarkably and dose-dependently downregulated the expression level of iNOS and COX-2. These results indicated that SPEH possesses strong in vitro anti-inflammatory activity.
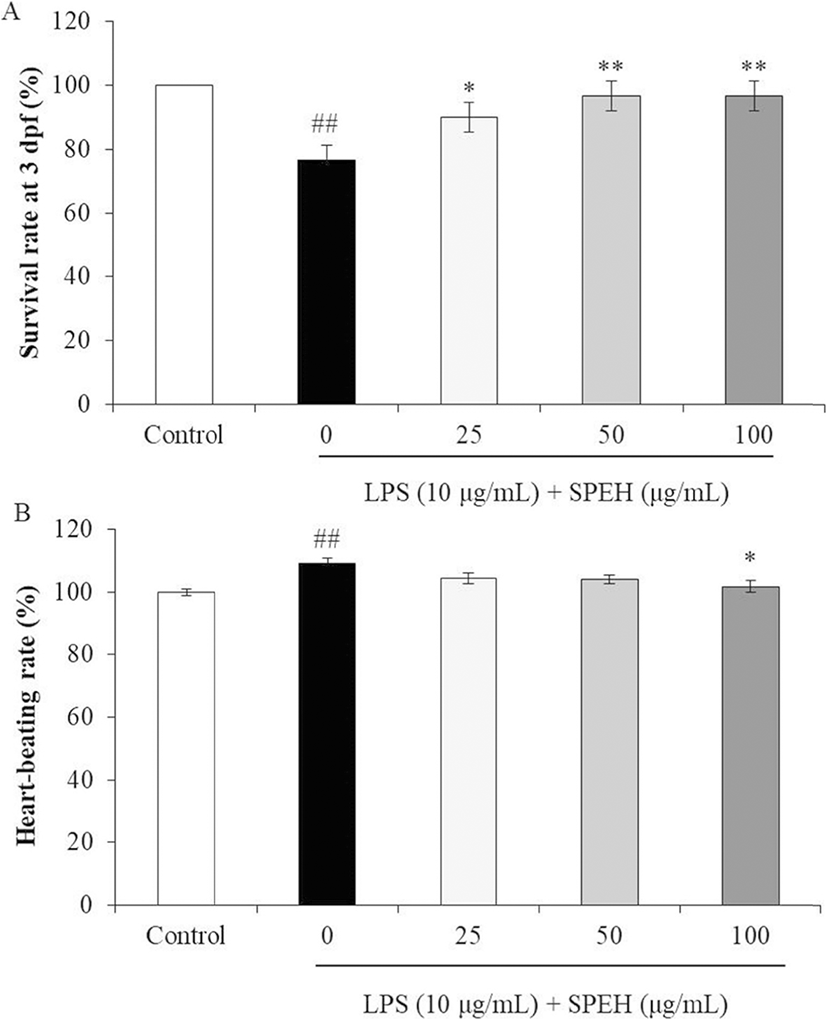
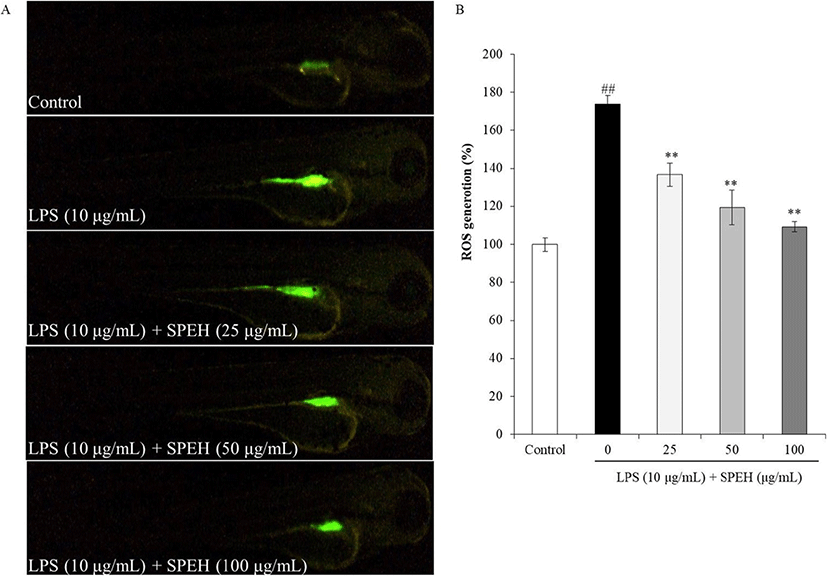
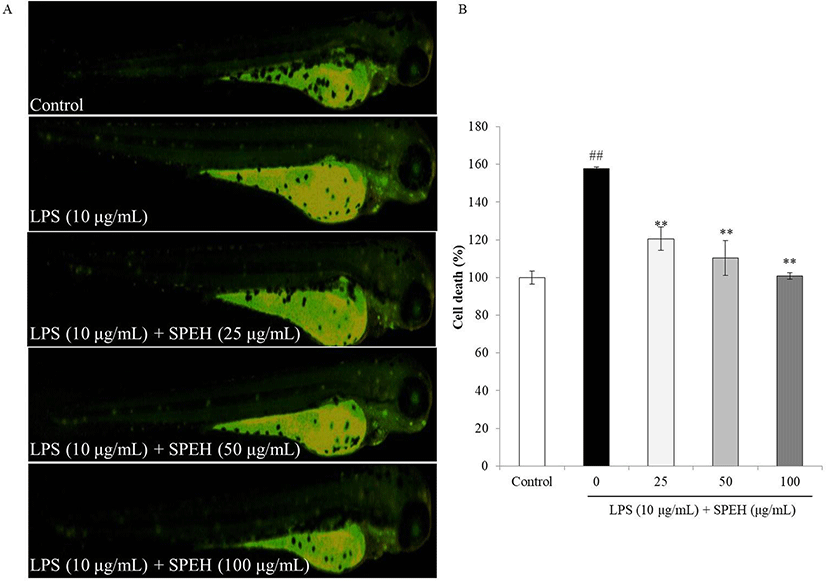
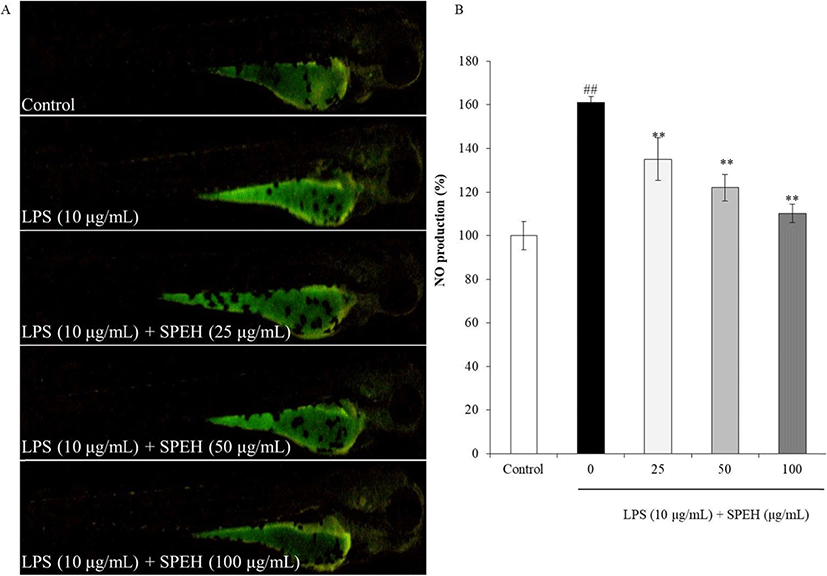
In vivo evaluation for anti-inflammation activity of SPEH was adopted in zebrafish due to its advantages such as similarity to mammals, short life span, and the ability of the female fish to produce a large number of eggs (Kishi et al. 2003; Ko et al. 2011). In the present study, we investigated the survival rate, heart-beating rate, ROS generation, cell death, and NO generation in zebrafish. As shown in Fig. 4a, while the survival rate of zebrafish was significantly reduced by LPS, it was remarkably and dose-dependently increased by SPEH-treatment. In addition, the heart-beating rate of LPS-treated zebrafish was significantly increased (112.93%) compared to non-treated zebrafish (100%) (Fig. 4b). However, it was restored to the normal level in the zebrafish treated with 100 μg/mL of SPEH (101.65%) (Fig. 4b). The ROS generation and cell death levels of SPEH-treated zebrafish were significantly and dose-dependently reduced compared to the zebrafish treated with LPS only (Figs. 5 and 6). Furthermore, SPEH pretreatment reduced 26.04, 39.08, and 50.88% of NO production in LPS-stimulated zebrafish pretreated with 25, 50, and 100 μg/mL, respectively (Fig. 7). These results indicated that SPEH possesses strong in vivo anti-inflammatory activity.
Conclusion
In the present study, we prepared a sterol-enriched fraction from Spirogyra sp. (SPEH) and evaluated its in vitro and in vivo anti-inflammatory activities in LPS-stimulated RAW 264.7 cells and zebrafish. The results indicated that SPEH possesses strong anti-inflammatory activity in both in vitro and in vivo models and has the potential to be developed as a healthcare product or a drug to treat inflammatory diseases.