Background
In recent years, Peru has become one of the main producers and exporters of fishmeal and fish oil; consequently, Peruvian aquaculture is expected to grow about 121% between 2016 and 2030 (FAO 2018). The production of rainbow trout (Oncorhynchus mykiss) has also been increased significantly lately, and in the Puno region alone, has reached ~ 43,290 t per year ([PRODUCE] Ministerio de la Producción 2017).
Trout are carnivorous, freshwater, predatory fish that require higher levels of protein and lipid from animal sources than other livestock species. Due to its high nutritional value, fish meal is generally included in feed. However, fish meal is relatively expensive and its production is constantly decreasing (Huntington and Hasan 2009). Therefore, evaluating new dietary protein sources from vegetables and animal origin for rainbow trout is important. Studies in rainbow trout have shown that it is possible to substitute raw materials, such as vegetables (Adelizi et al. 1998; Zhang et al. 2012; Daniel 2018; Ortiz-Chura et al. 2018) or other animal protein sources (Pfeffer et al. 1994; Steffens 1994; Pares-Sierra et al. 2014; Javaherdoust et al. 2019), in replace for fish meal without significantly affecting the nutrition composition and biological performance of the fish.
Rendered byproducts such as feathers and skins (collagen, elastin, and keratin) from domestic animal slaughterhouses are protein sources that could be used in fish feed (Cheng and Hardy 2002; Bureau 2006). However, the structure of the proteins and the disulfide bonds (S–S) of sulfur amino acids hinder digestion and limit the use of these byproducts. Thermal vapor pressure hydrolysis is a physical treatment that makes it possible to simplify complex molecules into simple components, improving the nutritional quality and value, as well as the availability of amino acids in animal byproducts (Moritz and Latshaw 2001). Feather hydrolysate has been proved to have high digestibility in rainbow trout (Bureau et al. 1999), and enzymatic treatment in the white shrimp improves amino acid utilization (Mendoza et al. 2001; Pfeuti et al. 2019).
The largest production of sheep (Ovis aries) and alpacas (Vicugna pacos) is in the Puno region of Peru. At the moment, the skins from sheep and alpacas do not have any commercial importance (Pacsi 2016), and interest in using these organic byproducts for animal nutrition has lagged by Peruvian producers. However, recently, the use of sheep and alpaca skin hydrolysates in feed rations for dairy cows, under natural hypobaric conditions, was beneficial for milk production and in reducing ration costs (Cahuascanco-Quispe et al. 2019). This result gives us to understand that we can also use these byproducts in rainbow trout feeding.
Therefore, a better understanding of the nutrient content and nutrient digestibility of the hydrolyzed skins of sheep and alpacas in juvenile trout feeding is very useful as the skins may be a less expensive nutrient source than fish meal. This research was conducted to determine the nutritional composition and apparent digestibility of dry matter, organic matter, crude protein, crude fat, and digestible energy of skin hydrolysates from sheep and alpaca (Pioval-2® meal) in juvenile rainbow trout.
Methods
Experimental procedures were carried out in the Animal Nutrition Lab of Veterinary Medicine and Zootechnics Faculty at the National University of the Altiplano, Puno, Peru, at an altitude of 3828 m (15° 49′ 29″ S, 70° 00′ 56″ O). The digestibility assay was performed in a sedimentation column water recirculation system (0.5 l/s) equipped with a closed water treatment system, gravel filter (STF Filter System® Leri Model 002737), activated carbon filter, biological filter (clays with nitrifying bacteria, Proline®), and UV filter (X-Ray UV Light Boyo®, China).
Sheared dry skins of sheep and alpacas obtained from a wool and fiber local commercial were cut, washed, and then hydrolyzed at 15 psi and 130 °C for 120 min in a 25 l capacity autoclave (All American®) equipped with a manometer, thermometer, and thermostat to control the temperature and pressure. The hydrolyzed skins of sheep (HSS) and alpacas (HSA) were then dried, ground, sieved, and stored until use.
The ingredients and chemical composition of experimental diets are shown in Table 1. Three diets (a reference diet and two experimental diets) were evaluated in triplicate. The reference diet was formulated based on the nutritional requirements for trout ([NRC] National Research Council 2011). The experimental diets were composed of 70% reference diet and 30% of either HSS or HSA, according to the methodology proposed by Glencross et al. (2007). The ingredients of the diets were mixed and then extruded at 95 °C (Extruder Khal® EE800, Germany) with a 4-mm pellet size.
1Non-digestible marker
2DSM Aquaculture Premix per kg of feed provided: vitamins A = 14,000 UI, D3 = 2800 UI, E = 140 UI, K3 = 8 mg, B1 (thiamine) = 18 mg, B2 (riboflavin) = 20 mg, Nicotinamide = 150 mg, pantothenic acid = 50 mg, B6 (pyridoxine) = 15 mg, biotin = 0.8 mg, folic acid = 4 mg, C (ascorbic acid) = 600 mg, B12 (cyanocobalamin) = 0.03 mg, choline = 600 mg; and Minerals: manganese = 40 mg, iron = 20 mg, zinc = 20 mg, copper 1.5 mg, iodine = 1.5 mg, selenium = 0.3 mg, cobalt = 0.15 mg, BHT (butylated hydroxytoluene) = 120 mg
3Analyzed by combustion (Mcal/kg DM)
A total of 450 juvenile rainbow trout from a commercial line (Troutlodge®, USA) were used. The fish had an initial mean weight of 60.0 ± 1.32 g (Alexander Mobba-Excell® SI-130) and a total mean length of 17.9 ± 0.93 cm (Ichthyometer, Aquatic Eco-Systems®). The trout was handled after being sedated with 20 mg/l tricaine methane sulfonate (MS 222, Argent Chemical Laboratories, Redmond, WA, USA). The fish were randomly distributed into tanks specially designed to measure the digestibility of their feed (Fig. 1) with an average loading density of 6.4 kg/m3. Each digestibility tank (500 l capacity) had a sedimentation unit for fecal collection adapted from Rodehutscord et al. (2000). The average water quality parameters were as follows: pH 8.4, temperature 11.5 °C (Peachimeter SI Analytics Lab 850®, Germany), and dissolved oxygen 6.2 mg/l (HI 9146 Hanna® Dissolved Oxygen Meter).
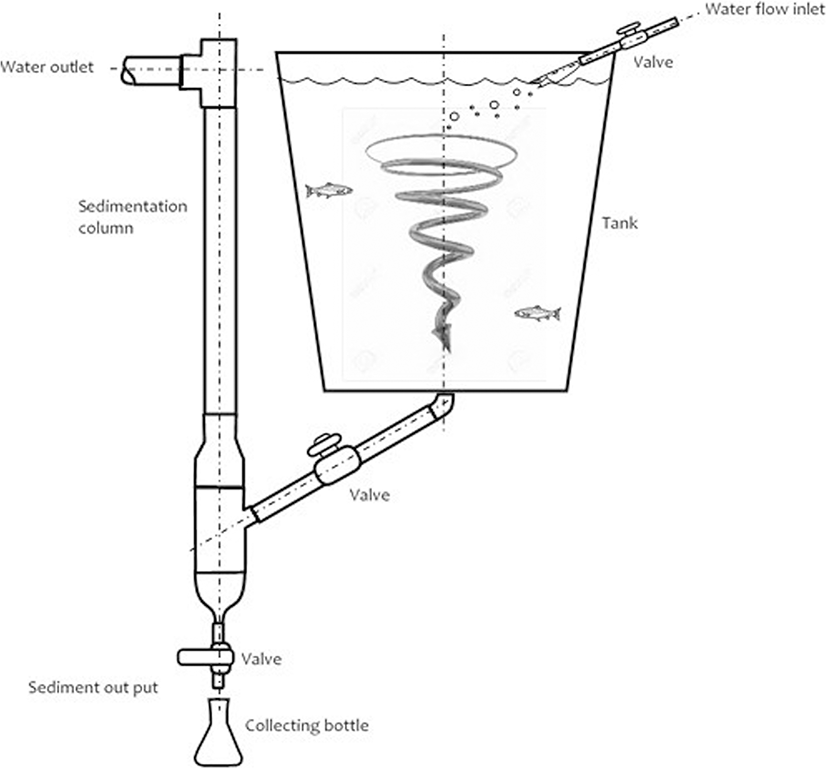
The apparent digestibility of the feed was determined by the indirect method of using an indigestible marker such as acid-insoluble ash (Hyflo Super Cel®) as previously described (Atkinson et al. 1984; Vandenberg and De La Noue 2001). Fish were given 7 days to become accustomed to the diet, followed by 7 days of fecal collection, and then, they were raised until 40 days to determine the performance of productive parameters.
The experimental diets were supplied twice a day (10:00 and 16:00 h). After feeding, all feed waste was removed from the system and collected directly from the sedimentation bottle. The experiment was performed under a natural light regime (range during experiment from 12:36 to 12:10 daylight hours).
The apparent digestibility of dry matter (DM), organic matter (OM), crude protein (CP), crude fat (CF), and digestible energy (DE) was determined using Eq. (1) proposed by Forster (1999).
where AD is the apparent digestibility (%), MD is the marker in the diet (%), MF is the marker in the feces (%), NF is the nutrient in the feces (%), and ND is the nutrient in the diet (%).
The apparent digestibility of DM, OM, CP, CF, and DE from the hydrolysates was estimated according to Eq. (2) proposed by Sugiura et al. (1998).
where ADi is the apparent digestibility of the ingredient under study (%), ADCt is the apparent digestibility coefficient of the diet evaluated, ADCb is the apparent digestibility coefficient of the reference diet (%), Db is the nutrients of the reference diet (%), Dt is the nutrients of the experimental diet (%), s is the proportion of the ingredient evaluated in the diet, and 1-s is the proportion of the reference diet in the experimental diet.
The DM, OM, CP, CF, gross energy (GE), and DE of the hydrolyzed skins of sheep (HSS) and alpacas (HSA) were determined. The ingredients, feed, and feces were analyzed according to the methodology of the [AOAC] Association of Official of Analytical Chemists (2011). Furthermore, GE was determined with a pump calorimeter (Parr Instrument 6772® USA). The non-digestible marker in diets and feces was determined according to the methodology proposed by Scott and Boldaji (1997).
The initial weight, final weight, daily weight gain, feed consumed daily, and feed conversion rate of trout were controlled from 0 to 40 experimental days.
Data analysis was performed using the analysis of variance (ANOVA) procedure in the SAS statistical program (SAS Institute Inc 2004). Mean values were compared using the LS mean test. Differences were considered significant at p < 0.05.
Results
The chemical composition and GE between HSS and HSA were significantly different before digestion (Table 2): OM (92.6 vs 95.2%, p < 0.001), CP (69.2 vs 76.7%, p < 0.01), CF (12.0 vs 7.0%, p < 0.001), ash (7.4 vs 4.8%, p < 0.001), and GE (5.79 vs 5.41 kcal/g, p < 0.001), respectively. But DM content does show not significant difference between HSS and HSA (94.9 vs 94.5%, p > 0.05).
Means with different letters in the same column differ significantly at p < 0.05
The apparent digestibility of DM and OM of the reference, HSS, and HSA diets were not different (Table 3). The reference diet did have a higher apparent digestibility than HSS and HSA for CP (92.5 vs 83.4 and 82.7%; p < 0.001), CF (92.2 vs 78.6 and 87.5%; p < 0.001), and GE (80.1 vs 73.3 and 74.0%; p < 0.001).
Means with different letters in the same row differ significantly at p < 0.05
1Expressed in Mcal/kg DM
Also, the apparent digestibility of DM (67.7 vs 69.1%), OM (66.9 vs 68.2%), CP (70.4 vs 70.1%), and DE (3.35 vs 3.24 Mcal/kg) were not different between HSS- and HSA-containing diets (p > 0.05), respectively. The apparent digestibility of CF was statistically significant between HSS and HSA diets (12.3 vs 48.0%; p < 0.001).
The productive parameters (initial weight, final weight, weight gain daily, feed consumed daily, and feed conversion rate) of trout were similar for all treatments (p > 0.05) during the experiment (Table 4).
Discussion
The OM content between raw HSS and HSA was significantly different (Table 2). Both hydrolyzed meals contained more OM than the range observed in fish meal (Gaylord et al. 2008; Lee et al. 2020). The lower OM content in HSS was directly related to a higher ash content present in minerals ([AOAC] Association of Official of Analytical Chemists 2011). HSS probably contained more external foreign material than HSA, this is due to the higher value of ash present. Also, ash content higher than normal values in raw materials suggests some type of contamination for presence of sand or other dirt (Salgueiro et al. 2010). The CP content was higher for HSA than HSS while HSS had a higher CF content than HSA. It is well documented by [NRC] National Research Council (2011) that high levels of CP are directly related to low levels of CF in raw materials (soybean meal solvent extracted vs soybean full fat extruded). The GE content was higher for HSS than HSA because higher levels of fats provide more energy to the feed. Fats contain approximately two times more calories than protein ([NRC] National Research Council 2011). These results confirm that the carcasses of South American camelids (alpaca and llama) are, in general, leaner than carcasses of sheep (Cristofanelli et al. 2005; Farid 1991). However, the GE content of both hydrolyzed meals was within the range reported in fish meal (Gaylord et al. 2008; Lee et al. 2020).
The CP content in both HSS (69.2%) and HSA (76.7%) was adequate for feed. Bureau (2006) proposed that rendered animal protein values that range from 66 to 85% indicate good processing. While lower values indicate insufficient hydrolysis, higher values indicate excessive processing, which would affect the availability of amino acids. Variations in the composition of proteins from animal sources are due to the variability in the quality of ingredients and processing conditions, such as cooking time, temperature, pressure, and storage conditions before and after treatment (Papadopoulos et al. 1985).
The apparent digestibility of DM, OM, CP, and GE were not different between the experimental diets or between the hydrolyzed skin meals. However, the apparent digestibility of CF was higher for HSA than HSS, which would be related to the lower content of saturated fats in raw HSA. In fact, more saturated fats are less digestible than less saturated fats or oils (Hua and Bureau 2009), although these differences in the digestibility of CF are of little economic importance, due to the low fat content of HSS and HSA (12% and 7%, respectively).
In general, the digestibility of DM (~ 80%) and CP (~ 90%) of fish meal in trout is 10% and 20% higher than the skin hydrolysates, respectively. Recently, Lee et al. (2020) reported greater differences in DM digestibility (86.6%) and CP (84.1%) for two different types of fish meal. This difference was due to the high nutritional quality of fish meal, characterized by its high biological value of protein and high content of essential amino acids and unsaturated fatty acids (Daniel 2018). Our results suggest that skin hydrolysates could partially replace fish meal in feed, with little to no change in the biological performance of the fish (Table 4).
Cho and Kaushik (1990) reported that hydrolyzed feather meal has higher digestibility of DM (75%) and lower digestibility of CP (58%) than HSS and HAS. Similar values were reported by Lee et al. (2020) for feather meal digestibility (DM 67.6% and CP 70.6%). Feather meal achieves less hydrolysis during heat treatment than what occurs in skin hydrolysates from sheep and alpaca. The quality of feather meal protein is also lower, since it is mainly composed of fibrous protein (keratin), while skin tissue contains mostly elastin and collagen as a protein source (Zhang et al. 2005).
The average DE value of HSS and HSA was 3.30 Mcal/kg and is lower than fish meal, poultry byproduct meal, and meat-and-bone meal (Lee et al. 2020). However, it was higher than that of hydrolyzed feather meal (Gaylord et al. 2008). The nutritional value of poultry byproduct meal is similar to fish meal (Bureau et al. 1999), and 20 to 25% can be included in salmonid diets without affecting the growth or feeding efficiency of fish (Steffens 1994) because it has a protein digestibility greater than 80% (Cheng and Hardy 2002). The protein availability and subsequent digestibility increases because of small polypeptide chains or short chains of amino acids that are produced during the hydrolytic process (Silva et al. 2017).
The digestibility of both HSS and HSA is acceptable and could be used as a partial replacement for fish meal. Sheep and alpaca skins are potential sources of elastin, collagen, and keratin discarded by the rural producers in Peru. These “waste” materials can be hydrolyzed into a source of available protein to be used in the feed of aquatic species.
Conclusion
This study represents the first work carried out using skin hydrolysates from sheep and alpacas for trout feed. Based on the results, skin hydrolysates of both slaughter animals have a high content of nutrients and a digestibility of macronutrients lower than fish meal but greater than feather meal. Alpaca and sheep skin hydrolysates (Pioval-2® meal) are cost-effective sources of nutrients and can also be used to improve the nutritive value of more economical feeds and are a viable alternative for feeding rainbow trout in Peruvian rural conditions.