Introduction
Seaweeds are considered as a traditional food in Asian countries. Chemical constituents obtained from seaweeds express various biological, functional, and nutritional activities. Terpenoids, flavonoids, essential fatty acids, vitamins, xanthophyll, proteins, and carotenoids are considered as valuable bioactive components in seaweeds (Neoh et al., 2016). Besides, seaweeds provide a plethora of health-promoting nutrients (Matanjun et al., 2009). Based on the previous studies, seaweeds are considered as a comparatively good source of certain vitamins, polysaccharides, and minerals (Darcy-Vrillon, 1993). In the economical stream, brown seaweeds are considered as the most important seaweed group (Chapman & Chapman, 1980). Moreover, brown seaweeds are abundant in bioactive compounds that can be used as functional ingredients for improving human health. The previous researches have reported their various bioactivities, such as anti-cancer, anti-inflammation, anti-diabetic, and antioxidant (Wijesinghe & Jeon, 2012). Normally, fresh seaweeds contain a large amount of water. Drying of seaweeds is used to reduce moisture retention and seaweed drying is an important and key step to ensure its nutritional value. Seaweeds that are properly dried can be kept for a long time (Naylor, 1976). Moreover, drying can be considered as an important post-harvest preservation method. However, the drying process causes some deformation of the vegetal and greater or lesser exposure of anti-oxidant compounds (Nguyen et al., 2016). Additionally, enzymatic degradation and microbial growth are down-regulated due to the drying process. Traditional drying techniques express some limitations, such as time-consuming, loss of quality in the plant, and loss of active ingredients (Fennell et al., 2004). The freeze drying method (FDM) is a convenient substitution for avoiding these limitations. FDM uses the dehydration process under low temperatures. Here, sublimation is used to remove ice by lowering the pressure. The quality of the product is high in this technique due to the low temperatures. However, the FDM is considered a comparatively high-cost method on an industrial scale. The cost of FDM is 5 times more than normal conventional drying methods. Further, the cost of energy is also high due to elevated energy consumption to complete the sublimation process (Ratti, 2001). According to the previous studies, the sun drying method has been widely and commonly used for economic reasons, seaweed studies, and phycocolloid industry. The sun-drying method generally expresses nearly similar nutritional quality to the FDM than other traditional drying methods. However, this method also contains limitations comparatively with the FDM such as depending on environmental factors and high labor-intensive (Chan et al., 1997).
On the other hand, recently hybrid hot-water Goodle dryer (HHGD: Korea Patent No. 1559558, 1613666, 1971728, 1935005) has been developed by King Ston located in Buyeo-gun, Chungcheongnam-do, Republic of Korea to avoid the above mentioned limitations. It could express various positive impacts on the seaweed drying process comparatively with other methods such as low cost, low energy consumption, low labor incentive, and ensure the nutrient composition of the seaweeds. HHGD is to use hot-water under the Korean traditional floor heating system (Goodle-Jang) and then this hot-water heats flat stone, which a far infrared irradiation emits from. Consequently, it names a “hybrid hot-water Goodle dryer”.
Hence, this paper aims to evaluate the effect of the hybrid hot-water Goodle drying method (HHGDM) on the nutritional composition and antioxidant activity of the selected brown seaweeds to allow the novel pathway for developing new nutraceuticals and functional food ingredients.
Material and Methods
Vero cells were purchased from the Korean cell line bank (KCLB, Seoul, Korea). Roswell Park Memorial Institute Medium (RPMI) and fetal bovine serum (FBS) were purchased from GIBCO (Grand Island, NY, USA). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 5,5-dimethyl-1-pyrroline N-oxide (DMPO), ferrous sulphate (FeSO4), hydrogen peroxide (H2O2), α-(4-pyridyl N-oxide)-N-tert-butylnitrone (POBN), 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), Hoechst 33342, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 20,70-20 70-dichlorodihydrofluorescein diacetate (DCFH-DA), and acridine orange, were purchased from Sigma, Aldrich (St. Louis, MO, USA) USA. All the other chemicals used in this study were of high purity grade.
The seaweed species were collected along the shore of Jeju Island, South Korea from February to March 2019. Seaweeds, Padina arborescens (PA), Sargassum autumnale (SA), Sargassum thunbergii (ST), and Ishige okamurae (IO) were used for drying by HHGDM and FDM. All the samples were washed thoroughly to remove salt, attached epiphytes, and sand.
Each chopped seaweed sample was divided into two portions, and HHGDM and FDM were used to dry each portion. The first portion of each seaweed species was frozen in a –80°C freezer for 24 h and dried in a freeze-drier for 2 days. The second portion of each seaweed species was dried using HHGDM. The conditions are heating temperature 60°C, inner humidity 12%, carbon mat temperature 70°C with running vortex and suction blower.
HHGDM and FDM samples were ground into fine particles. 200 g of each subsequent powder was extracted with 2 L of 70% ethanol at room temperature under 100 rpm speed shaking. The above-mentioned steps were repeated three times and the resulted solutions were filtered using Whatman No. 4 filter papers (Whatman, Kent, UK), and a rotary evaporator was used to concentrate the subsequent solvents of filtered ethanol-soluble fractions. The solvent-free seaweed extracts were obtained by HHGDM and FDM. DMSO was used to dissolve the ethanolic extracts of seaweeds from HHGDM and FDM.
The general composition of seaweeds dried from HHGDM and FDM were analyzed according to the AOAC standard 2005 standards (AOAC International, 2005). The ash content of seaweed samples from the two different drying methods were measured by drying in a furnace at 550°C for 6 h (Sanjeewa et al., 2019). Kjeldahl digestion method was used to determine the protein content of seaweed samples from HHGDM and FDM. The lipid content was measured by the Soxhlet method, hexane with di-ethyl ether were used as the solvent (Soxtec 2050; FOSS Analytical, Hillerød, Denmark) (Kim et al., 2018). For the chemical composition of the 70% ethanol extract of the seaweed samples from HHGDM and FDM, the crude proteins were measured by a commercially available BCA protein assay kit (Pierce Company, Massachusetts, USA). Bovine serum albumin was used as the standard and each measurement was triplicated. The phenol contents were measured by Folin-Ciocalteu’s method. Gallic acid was used as a standard (Chandler & Dodds, 1983). The Phenol-sulfuric acid method was used to measure the polysaccharide content. Glucose was used as the standard (Kim et al., 2018). The intercellular antioxidant activities of the seaweed extracts were analyzed using three radicals. Sample concentration was 2 mg/mL. Phosphate saline buffer was used as the control. DPPH, alkyl and, hydroxyl radical activities were measured respectively according to the previously reported methods (Nanjo et al., 1996; Hiramoto et al., 1993; Finkelstein et al., 1980). Electron spin resonance (ESR) spectrometer was (JESFA200; Jeol, Tokyo, Japan) used to evaluate the radical scavenging ability of seaweed extracts. The instrument configurations of modulation frequency 100 kHz, central filed 3475 G, modulation amplitude 2 G, gain 6.3 × 105, and temperature 298 K. 5 mW, 10 mW, and 1 mW microwave powers were used to DPPH, alkyl, and hydroxyl respectively.
The cytotoxicity of the seaweed extracts was determined via MTT assay (Sanjeewa et al., 2017). The cells were seeded with 1 × 105 cells/mL in 96 well plates. The seeded cells were incubated for 24 h. The series of seaweed extract concentrations (12.5 µg/mL, 25 µg/mL, 50 µg/mL, 100 µg/mL, and 200 µg/mL) were used to treat the cells. Then the cells were treated with MTT solution prepared in PBS after 24 h and provide an incubation period for make formazan crystals. The formed formazan crystals were dissolved in DMSO. The absorbance was measured by SynergyTM HT, Vermont, USA, plate reader. The absorbance values were expressed as a mean percentage value relative to controls.
Three seaweed concentrations were used to evaluate the intracellular antioxidant effect. Vero cells were seeded (1 × 105 cells/mL) in 96 well plates. Cells were treated with samples after 24 h incubation period and co-treated with 1 mM H2O2 and 10 mM AAPH. The cell viability was evaluated after 24 h incubation by MTT assay.
DCFH-DA assay was used to determine the intracellular ROS scavenging ability of the seaweed extracts (Yang et al., 2011). The Vero cells (1 × 105 cells/mL) cultured in 96 well plates were used to treat by samples (12.5 µg/mL, 25 µg/mL, and 50 µg/mL) from different drying methods. The sample treated cells were treated with H2O2 and AAPH (1 mM) and provide 1–3 h incubation period. DCFH-DA (25 µg/mL) 10 µL was added to each well and provided another 10 min incubation period. Synergy HT multi-detection microplate reader (Bioteck, Winooski, VT, USA) was used to measure the fluorescence at an excitation and an emission wavelength of 485 and 530 nm.
The apoptotic body formations of Vero cells were characterized using Hoechst 33342 nuclear staining method by staining nuclear fragmentation and chromatin condensation of cells (Lee et al., 2015). The cells were seeded in 24 well culture plates. The seeded cells were treated by seaweed extracts from different drying methods after 24 h incubation period. The sample treated cells were stimulated by 1 mM H2O2 after the incubation period. Then each well was treated with 5µL of Hoechst 33342 stain (10 µg/mL) after 24 h incubation period (Fernando et al., 2018). The fluorescence images were taken after 10 min incubation by the fluorescence microscope equipped with a Cool SNAP-Pro color digital camera (Olympus, Tokyo, Japan).
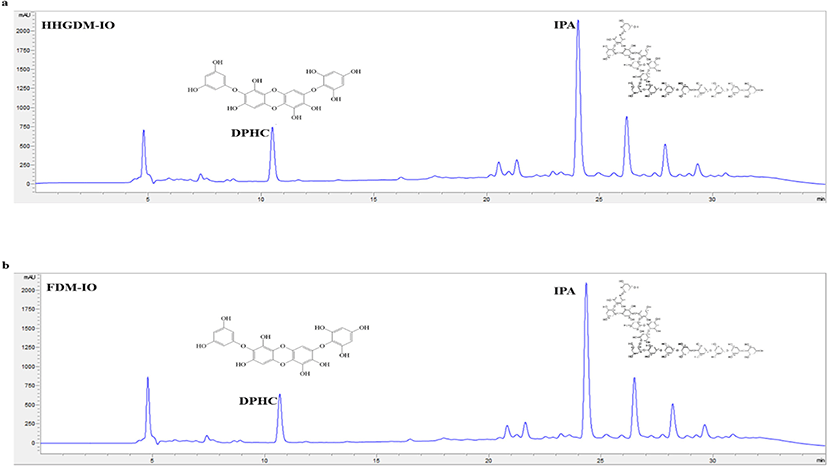
The analysis was performed using Semi-preparative C18 (Agilent proshell 120, 4 µm, 4.6 × 100 mm) column connected to a 1220 Infinity IILC HPLC system. The system was equipped with a UV/Vis detector. The HPLC grade solvents (Burdick & Jackson) were used for analysis. Distilled water was used as a mobile phase with 0.01% TFA (A) and acetonitrile with 0.01% TFA (B). The temperature of the column was maintained at 35°C. The gradient elution was performed at 0–30 min 69:31; 30–40 min 0:100 with flow rate 0.3 mL min and the ultraviolet absorbance was detected at 230 nm.
Adult zebrafish were purchased from Seoul Aquarium, Korea. The acrylic tanks were used to maintain zebrafish under controlled environmental conditions (12/ 10 h light/ dark cycle with 28.5°C). Fish were fed 6 days per week and 3 times per day by live brine shrimps with tetramine flake. The embryos were obtained by natural spawning which was stimulated with lights on conditions and collection was completed within 30 min.
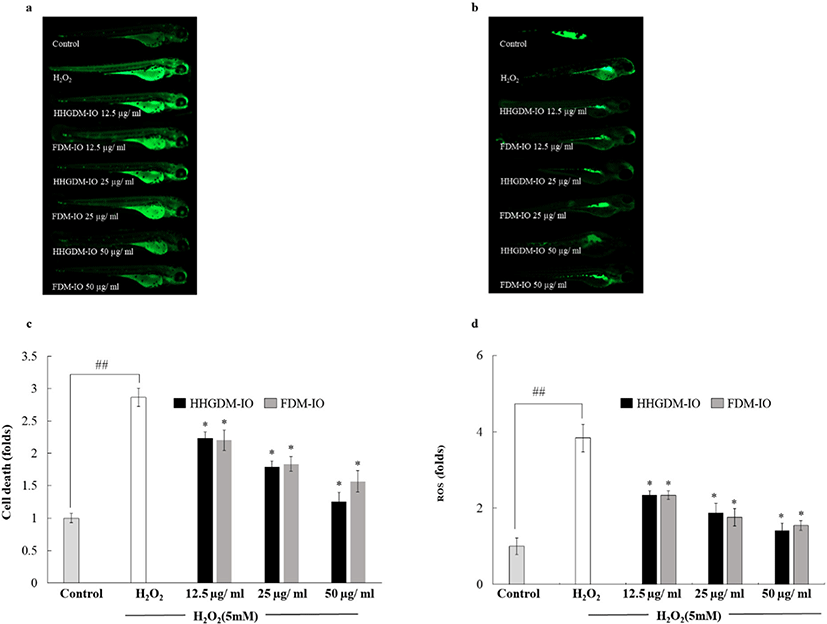
The healthy zebrafish embryos were obtained after 7–9 h post-fertilization (hpf). The selected embryos were transferred to embryo media in 12 well culture plate and periodically monitored. The transferred embryos were treated with IO from HHGDM (HHGDM-IO) and FDM (FDM-IO) (12.5 µg/mL, 25 µg/mL, and 50 µg/mL) and incubated for 1 h and stimulated with 5 mM H2O2 (24 h) for evaluating the ROS level and cell death using fluorescence dyes.
The heartbeat rate was measured at 48 hpf to evaluate the toxicity of the sample (Choi et al., 2007). The heartbeat rate of both atrium and ventricle contractions within 3 min were measured under the microscope and the average heartbeat rate per min was presented as the final result. Oxidative sensitive fluorescent probe dye, 2, 7-dichlorodihydrofluorescein diacetate (DCFH-DA) (20 µg/mL) was used to analyze the H2O2 induced ROS generation in zebrafish embryos according to the previously described method with slight modification (Kang et al., 2013). Cellular peroxides cause further oxidation of DCDH-DA to fluorescence compound dichlorofluorescein (DCF) (Rosenkranz et al., 1992). Acridine orange staining (7 µg/mL) was used to detect cell death in live embryos (Kang et al., 2013). The fluorescence images were taken by the fluorescence microscope equipped with a Cool SNAP-Pro color digital camera (Olympus, Tokyo, Japan). The obtained images were quantified by ImageJ 1.49v (National Institutes of Health, Bethesda, MD, USA).
Results and Discussion
The drying of the seaweed is a highly important factor for the marine industry and offers possibilities for isolation, development of compounds, and novel products to consumers. In recent years, the seaweed industry has used many different drying methods with advantages and limitations. The present study reveals that HHGDM is a possible solution to avoid these limitations.
Drying processes make the changes of seaweeds such as shrinkage and crispy nature which cause easy grinding to produce the powder. This is useful for further extraction and analysis. The drying method has an impact on nutrient composition and bioactivity of seaweeds, depending on their drying conditions (Kyriakopoulou et al., 2013). In general, several drying methods are used for seaweed dryings such as FDM, sun drying, oven drying, and vacuum drying (Neoh et al., 2016). However, each drying method contains advantages and disadvantages such as cost, capacity, and environmental factors. Therefore, the requirement of the drying method which can ensure the nutritional value of seaweeds after drying with low-cost is an important factor for the industrial sector. HHGDM is a comparatively low-cost method that uses low temperature for drying. This study was conducted to evaluate the quality of the dried seaweeds from HHGDM in proximate compositions and antioxidant activities by comparing with FDM.
The proximate compositions of seaweeds from the two different drying methods were analyzed by AOAC standard method (AOAC International, 2005). The results are summarized in Table 1 and Table 2. Among the dried seaweeds obtained from the two different drying methods, there was no significant difference in the proximate compositions including lipid, polysaccharide, protein, ash, and moisture content between HHGDM and FDM. Moreover, the yields as well as the contents of proteins, phenols, polysaccharides, sterols for the 70% ethanol extracts from HHGDM and FDM also did not express any significant difference. The moisture content of the dried samples is an important factor. Hence, the moisture content was used to evaluate the final moisture retention in dried seaweeds. All seaweed samples from HHGDM and FDM were dried until constant weight. The final moisture content of seaweed samples from HHGDM and FDM were 8.98%–11.95% and 8.15%–10.96%, respectively. This result reflects HHGDM can effectively remove moisture from the seaweeds like the FDM.
Phenolic compounds are widely accumulated in the cell wall. Moreover, other compounds such as flavonoids and lignin are accumulated in vacuoles. Phenolic compounds express their antioxidant ability due to their redox properties. The preservation of phenolic compounds is based on drying conditions (Chan et al., 2015). According to the results of Table 1, the phenolic content of dried seaweed from HHGDM was conserved without any significant difference with FDM. Moreover, this reflects the drying conditions of HHGDM do not express the negative effect on the phenolic content comparatively FDM. Antioxidants are substances that can significantly delay or inhibit the oxidation of the substrate even present in a low concentration compared to the oxidizable substrate (Halliwell & Gutteridge, 1985). Free radical scavengers are considered as antioxidants that are capable to scavenge ROS. These ROS cause modifications of lipids, proteins, and DNA that cause various diseases such as arthritis, diabetes, pulmonary dysfunction, and inflammatory disorders (Frölich & Riederer, 1995). The different levels of oxidative sequence can be affected by antioxidants. Moreover, antioxidants can inhibit free radical chain reaction by delaying the free radical formation or competing with existing radicals and removing those radicals from the reaction medium (Winata & Lorenz, 1996). Brown seaweeds contain numerous bioactive compounds such as phenolic compounds that exhibit antioxidant activities (Wijesinghe & Jeon, 2012). Therefore, drying conditions are important to conserve the antioxidant ability of the dried seaweed. Table 3 shows the primary screening results of the intercellular antioxidant ability of the 70% ethanol extracts. In the present study, DPPH, alkyl, and hydroxyl radicals were used to evaluate the antioxidant ability of four brown seaweed extracts. Moreover, the IC50 values of the seaweed extracts between HHGDM and FDM did not express any significant difference. According to the results, IO recorded the highest DPPH, alkyl, and hydroxyl radical scavenging activity.
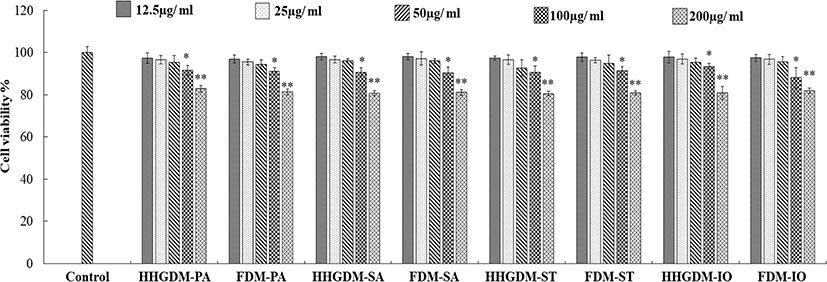
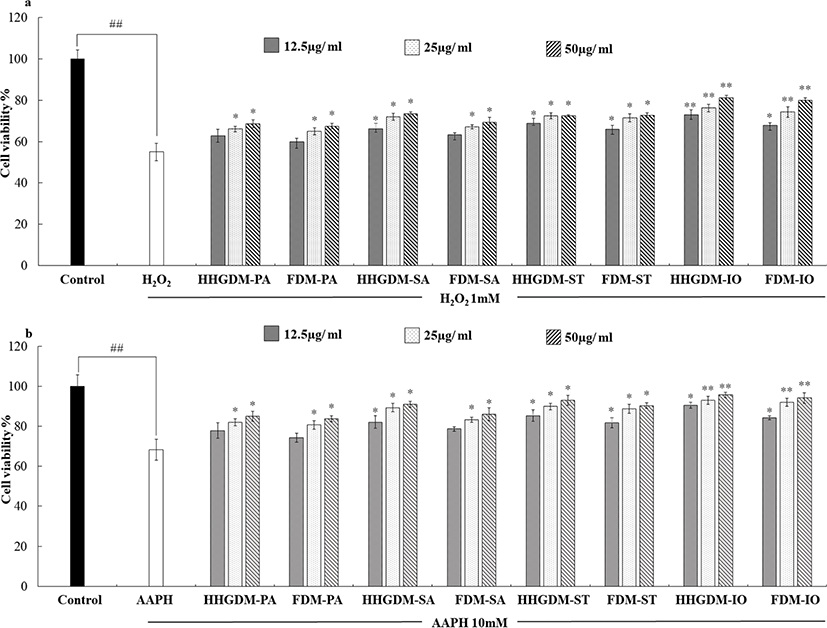
The cytotoxicity of the seaweed extracts was evaluated in Vero cells using MTT assay (Fig. 1). The five concentrations (12.5 µg/mL–200 µg/mL) were used to determine the cytotoxicity. According to the results, three concentrations did not express significant down-regulation of cell viability. Therefore, 12.5 µg/mL–50 µg/mL concentrations were selected for consecutive study. The Vero cells were stimulated with 1 mM H2O2 and 10 mM AAPH to evaluate the intracellular antioxidant activity of the seaweed extracts. The MTT results of the seaweed extracts from HHGDM expressed a significant and dose-dependent up-regulation in cell viability than the positive control groups. Moreover, these results did not express any significant difference with FDM. These results implied that the extract from dried IO seaweed obtained by HHGGM exhibited strong intracellular antioxidant activities and it was further confirmed that drying conditions do not negatively effect on nutrient composition of seaweed (Fig. 2).
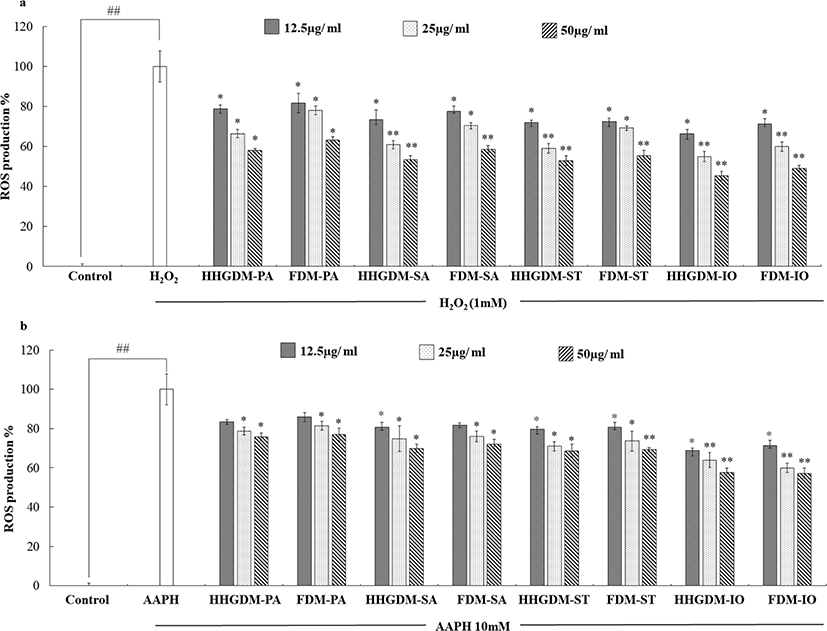
DCFH-DA assay was used to analyze the ROS scavenging ability of the 70% ethanol extracts of seaweeds (Fig. 3). Cells absorb DCFH-DA and cellular esterases convert DCFH-DA into non-fluorescent DCFH. The intracellular ROS and other peroxides convert non-fluorescent DCFH into fluorescent DCF that can be detected under an excitation wavelength of 485 nm and an emission wavelength of 530 nm (Rastogi et al., 2010). According to the results, the ROS scavenging activities of the seaweed extracts from HHGDM expressed a significant ROS scavenging ability in a dose-dependent manner and there was no significant difference when comparing to FDM. Interestingly, IO indicated elevated results in each parameter than other sea weeds, because of that, IO was selected for further studies.
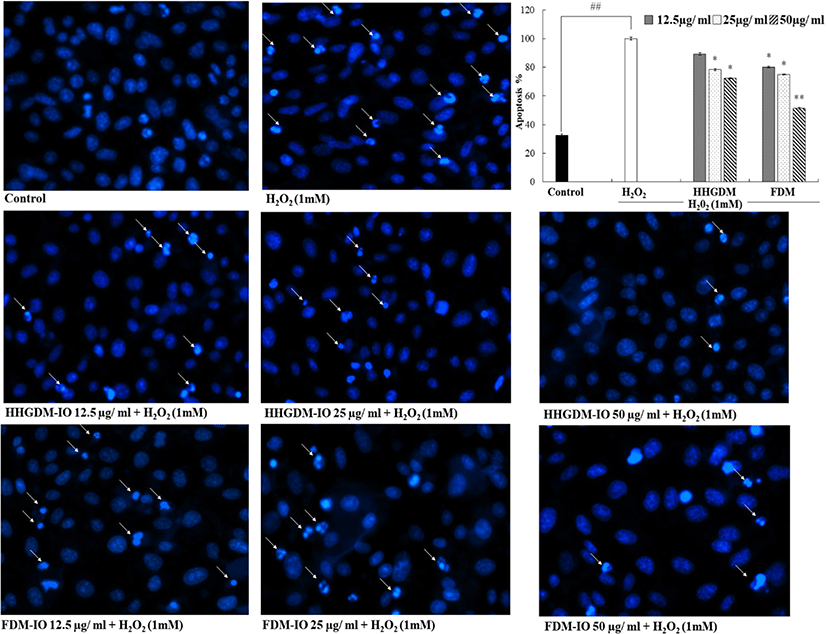
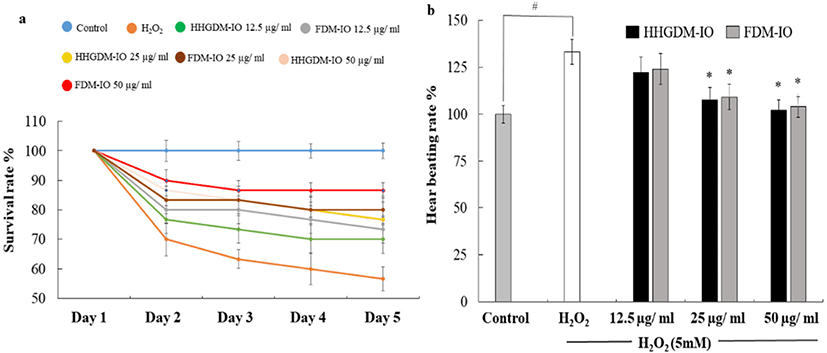
HHGDM-IO and FDM-IO were used to analyze the regulation of apoptotic body formation. The apoptotic bodies were visualized using Hoechst 33342 fluorescent dye. The apoptotic bodies are determined based on the nuclear morphology of the cells. Nuclei which are homogeneously stained consider as viable cells. The chromatin condensations or fragmentations are considered as apoptosis (Ahn et al., 2015). According to the results, HHGDM-IO significantly and dose-dependently reduced the apoptotic body formation (Fig. 4). Moreover, there are no significant differences between HHGDM-IO and FDM-IO in the down-regulation of apoptotic body formation. The zebrafish experiments were performed as an in-vivo model for further evaluations and comparison between the two different drying methods with IO extracts. The survival rate of zebrafish was significantly down-regulated than control after exposure to 10 mM H2O2. The survival rate was 56.07% on the 5th day after treating H2O2. However, zebrafish, pre-treated with HHGDM-IO showed a dose-dependent and a significant up-regulation of the survival rate than the H2O2 treated group. The heart beating rates of zebrafish exposed to 10 mM H2O2 were significantly increased than the control. Moreover, the elevated heart beating rate was down-regulated by the treatment of HHGDM-IO in a dose-dependent manner (Fig. 5). Acridine orange staining method was used to evaluate the cell death of zebrafish caused by H2O2 oxidative stress. The up-regulated cell death in zebrafish which was exposed to H2O2 was declined by seaweed extract treatments. DCFH-DA staining method was used to determine the intracellular ROS levels. The up-regulated ROS level with H2O2 was declined by HHGDM-IO significantly. Moreover, the interesting factor was no significant difference in the above results between HHGDM-IO and FDM-IO (Fig. 6). In the HPLC analysis, the 70% ethanol extracts obtained from the IO seaweed dried by HHGDM and FDM showed a similar main peak of diphlorethohydroxycarmalol (DPHC) and Ishophloroglucin A (IPA) according to the results, a peak with 10.5 min and 24.04 min retention time. It was observed that the major active compounds showed no significant difference between HHGDM-IO and FDM-IO (Fig. 7). This reveals that HHGDM could be a suitable drying method for ensuring the nutrient composition of seaweed. As a low-cost drying method, HHGDM expressed a minimum effect on the nutrient composition of dried seaweeds. This leads to the conservation of biological activities in dried samples such as antioxidant activity. The dried output of HHGDM did not show a toxic effect on cell-based experiments and the yield also wasn’t expressed a significant difference from the FDM. The considering factors reveal the potential of this method at the industrial level.
Conclusion
General composition results of the dried brown seaweeds and their 70% ethanol extracts between HHGDM and FDM revealed no difference in all the tests and bioassay evaluations. This reflects the drying method with HHGDM does not contain adverse effects on the nutritional composition of dried seaweeds. Moreover, these results were supported by intracellular and intercellular ROS scavenging activities, down-regulation of apoptotic body formation, in-vivo experiment, and HPLC analysis results. The FDM provides better nutritional quality to seaweeds, but the operating cost is higher and the drying capacity is lower than the other methods such as sun drying. However, the sun-drying method also depends on weather conditions, day lengths, and high intensive labor. From the results, HHGDM could be an alternative drying technique with a lower cost, lower labor but preserving valuable nutrients. Moreover, HHGDM appears to be the most appropriate drying method in industrial-scale productions.
