Introduction
Behavioral analyses are important for understanding various physiological aspects of an animal’s response to environmental changes as well as their health status. Aquatic animals are a good model for monitoring animal behavior, as chemical cues are spread by diffusion in the more homogeneous water medium. Fish detect myriad environmental signals that affect their behaviors, such as foraging, courtship, schooling, attraction, and aggression (Firestein, 2001; Kermen et al., 2013). Underwater acoustic cameras and vision-based tracking systems have been employed to monitor such fish movements in the aquatic environment (Kane et al., 2005; Kuklina et al., 2012; Niu et al., 2018). Although the former method is more suitable for analyzing behavior in large-scale experiments (Jang et al., 2016), video tracking is more convenient for detecting chemical cues that elicit a behavioral response in a smaller model system.
Rock bream, Oplegnathus fasciatus, is a marine fish that lives in rocky coastal areas off Korea. It is a popular species of high economic value, with interest in this species increasing for aquaculture production as well as for fishing. Some studies have investigated the physiology of rock bream associated with temperature and photoperiod, but relatively little is known about its physiology in terms of detecting chemicals present in the environment. Various materials, including squid, krill, and worms, are used as bait for rock bream fishing, although there has been an increasing demand to develop more efficient and environmentally friendly alternatives. It is important to find such materials that will attract rock bream efficiently but can be obtained at a lower cost.
Sea urchins are spiny marine echinoderms belonging to the class Echinoidea and considered a model organism for early development. One species, Strongylocentrotus nudus, is predominantly distributed along intertidal and subtidal sea-bottom (Agatsuma, 2007) areas off the northeast Pacific coast. Sea urchins play a critical role in maintaining the balance between corals and algae (Harrold & Pearse, 1987; Lang & Schroeter, 1976; Lawrence, 2007; Valentine & Heck, 1999). In particular, a high-density population of sea urchins overgrazing attached seaweeds has been identified as one of the most destructive factors in fish breeding grounds. Therefore, controlling the number of sea urchins that invade seaweed areas is a challenge in protecting the environment, including the reinforced marine forest off the eastern coast of Korea. Some efforts have been made to remove sea urchins from the area, resulting in a disposal problem as tons of collected sea urchins have to be dealt with. This could be alleviated by finding an efficient way to use or recycle the sea urchin biomass. Sea urchins with eggs have been used as food in Asian countries, but most of the sea urchins are thrown out. As many sea urchins are found in marine forest areas where the omnivorous rock bream is present, their potential use as bait for rock bream fishing has been suggested. Thus, in this study, we compared the efficacy of a sea urchin extract to that of other foods, including rock worm, bait worm, krill, and barley kernel, for use as fishing bait by monitoring the behavioral response of the rock bream upon exposure to these cues.
Materials and Methods
Rock bream (weight 8.8 ± 1.8 g; length 7.4 ± 0.6 cm) were obtained from a commercial aquaculture facility. The fish were maintained in a tank with 33 ± 2 ppt recirculating sea water at 20 ± 2°C and fed 3% of their body weight with commercial fish feed. The sea urchins were obtained from eastern coastal areas of Korea with the help of the Korea Fisheries Resources Agency. Rock worms (Marphysa victori) were provided by the Fishery Technology Center of PKNU (Goseong, Korea). Bait worms, krill, and barley kernels (bait grade) were obtained from a local market that sells bait in Busan, Korea. Handling and treatment of the experimental animals were conducted in accordance with governmental regulations and relevant standards. A Y-shaped acrylic tank (size of a single-arm: 48 cm L × 33 cm W × 28 cm H; Fig. 1A) was used to analyze the efficacy of the test foods for attracting juvenile rock bream (Jang et al., 2017). The housing and maintenance of the animals conformed to the regulations of The Institutional Animal Care and Use Committees of PKNU.
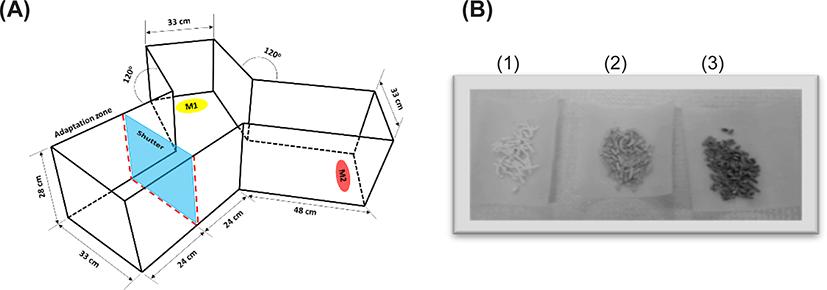
The internal tissues dissected from the outer shell of the sea urchin were tested for their ability to attract the fish along with worms and krill. Samples held at −80°C were homogenized followed by freeze drying. A cellulose pellet (10% corn flour in cellulose) was prepared by mixing 25.2 g of cellulose with 2.8 g of corn flour in 65 mL of water, and the mixture was passed through a 10-mL disposable syringe without a needle (Duminda, 2020). After partial drying, the pellets were cut into 1-cm lengths and air-dried. Freeze-dried samples (0.2 g) were resuspended in 1 mL of water and adsorbed onto a 1-g cellulose pellet followed by air drying (Fig. 1B).
The fish were maintained in a rectangular tank and provided commercial feed (1% of body weight) daily but starved 1 day before the experiment. To monitor behavior, five randomly selected fish were acclimated for 15 min in the adaptive zone, which was separated by an acrylic shield from the other arms in the Y-shaped tank. The fish were exposed to food materials positioned near the ends of the other arms, as shown in Fig. 1A. Pellets containing a known amount of test material were inserted into an empty tea bag, which was then placed in a plastic container (11 cm × 8 cm × 4 cm) with slits in the lid. Plastic containers, each containing a test food, were positioned at the ends of the other arms. Fish movements were monitored for 15 min upon exposure to the test materials by removing the separating shield (Duminda, 2020).
The behavioral responses of rock bream were recorded by a camera fixed on top of the tank. Movement patterns were analyzed using EthoVision XT 10 (Noldus Information Technology, Leesburg, VA, USA) video tracking software. The analog video signals captured by the camera were digitized by a frame grabber for automated analysis as described previously (Noldus et al., 2001). Movement tracks were analyzed using heat maps and the occurrence frequency of fish detected in the designated zones around the test materials in the experimental tank as well as their duration of stay were recorded.
The experimental data are presented as the mean ± SD, and the statistical analysis was performed using the PASW statistical program ver.18 (IBM, Armonk, NY). Differences among experimental groups (p < 0.05) were examined using one-way analysis of variance and the paired t-test. A p-value < 0.05 was considered to indicate significance.
Results
Video tracking systems have been used previously to monitor the behavioral responses of animals. To explore whether this system can be applied to investigations of aquatic animals, we tested this system by exposing rock bream to various test materials, including extracts of sea urchins and other bait candidates. Cellulose pellets containing amounts ranging from 0.1 g to 1.0 g of freeze-dried sample were prepared to determine the optimum amount that elicited a response. To examine the effect of a chemical diffused from the pellet, pellets containing the test materials were first covered with a tea bag, which was then placed in a plastic cage with slits in the lid to avoid any visual effect. For each trial, five fish of similar size were acclimated at one side of the designated arm in the Y-shaped tank, and their behaviors, such as quick movements with frequent rotation, frequent pecking at the ground and corners, and feeding movements, were observed to confirm the normal behavior of the fish in the tank. The fish movements were recorded for 15 min upon exposure to the pellets in the cages located at the ends of the opposite arms after removing the shield. The level of preference toward each test material was analyzed using a heat map and by assessing the duration of stay, occurrence frequency, and other behavioral parameters.
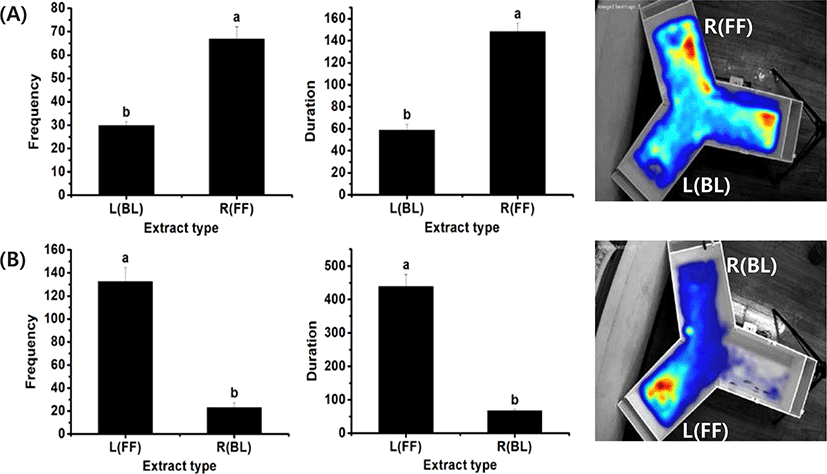
To test whether the system can be used to compare the attraction efficacy of each food type, control experiments were carried out with two pellets containing extracts of fish feed and barley kernel, which were positioned at each end of the test arms. Preliminary experiments indicated that 0.2 g of freeze-dried rock worm and sea urchin material induced attraction relative to the control pellets (data not shown). Preference toward each material was determined by the occurrence frequency, duration of stay, and the heat map of rock bream occurrence in the designated area in each arm with test material. The results showed a higher frequency of rock bream detected in the zone around commercial feed (44.2 ± 24.2) compared to that around barley kernel (17.3 ± 9.4), indicating a clear preference for the feed. A similar result was obtained based on duration of stay (120.7 ± 56.7 vs. 39.3 ± 16.3). To further confirm the result, another set of experiments was carried out with the same materials, but these were placed in opposite arms. The results also showed a higher frequency (93.5 ± 40.7) and duration of stay (305.4 ± 136.4) in the zone around the fish feed compared to barley (frequency 29.6 ± 7.9; duration 89.9+ 24.8). The fish feed was a better attractant as shown by the heat map analysis; a higher intensity of red was observed than in the heat map for barley (Fig. 2), and an opposite pattern was observed in the reverse-orientation experiment. The results showed a consistent pattern for a stronger preference toward commercial feed compared to barley regardless of their positions in the tank, indicating that this video system can be used to compare the degree of attraction exerted by candidate materials.
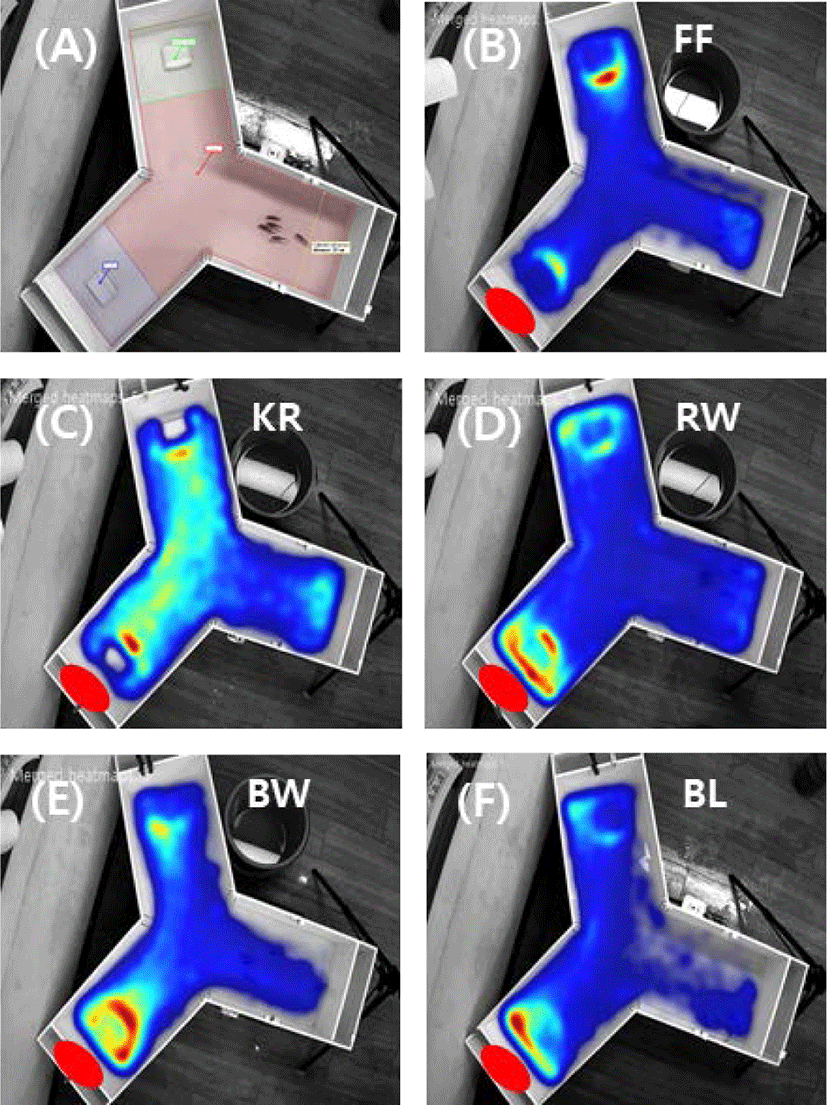
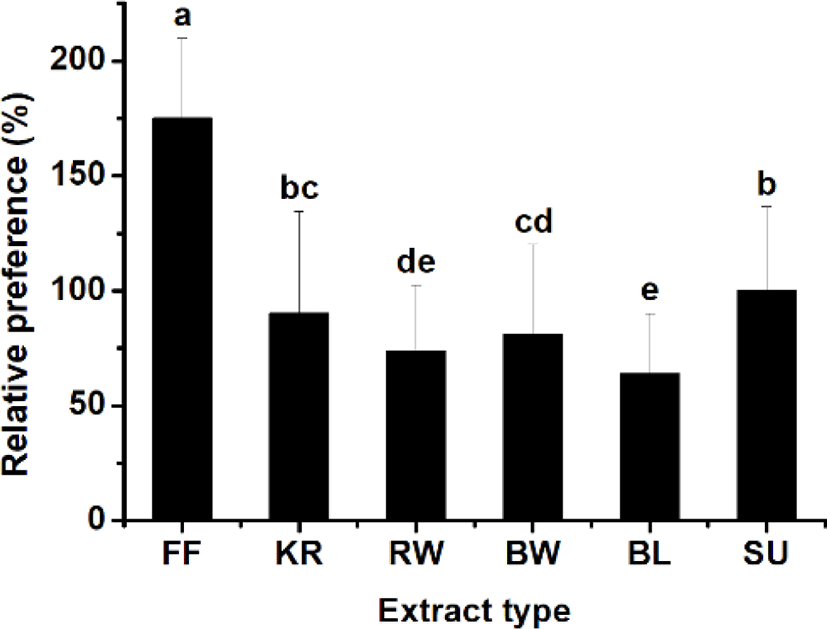
The attraction efficacy of the materials containing worms and krill, which are commonly used as bait for fishing, was tested. It was of particular interest to explore the potential attraction efficacy of sea urchin compared to the other materials, which include fish feed, krill, bait worm, rock worm, and barley. An initial experiment was carried out in a rectangular tank (data not shown), and the Y-shaped tank was used to compare the relative preference between materials located at each end of the two test arms in the tank. Experiments were carried out with a pellet containing sea urchin extract on one side together with a pellet containing another material on the other side. Fig. 3 shows the heat maps reflecting rock bream occurrence around the test food materials, indicating a stronger preference toward the sea urchin extract than the other materials, including krill, worms, and barley, except the commercial fish feed, which was most highly preferred. The relative preference toward each test material was calculated from a comparison of the frequency of fish detected in each zone. The results showed a relative preference toward commercial fish feed, krill, bait worm, rock worm, and barley of 175 ± 34.9%, 90.1 ± 44.2%, 81.1 ± 39.1%, 73.7 ± 28.9%, and 63.9 ± 25.9%, respectively, compared to sea urchin, whose preference level was set to 100% (Fig. 4). These results indicated that the highest preference (p < 0.05) was for the commercial fish feed followed by sea urchin. The lowest response was to barley.
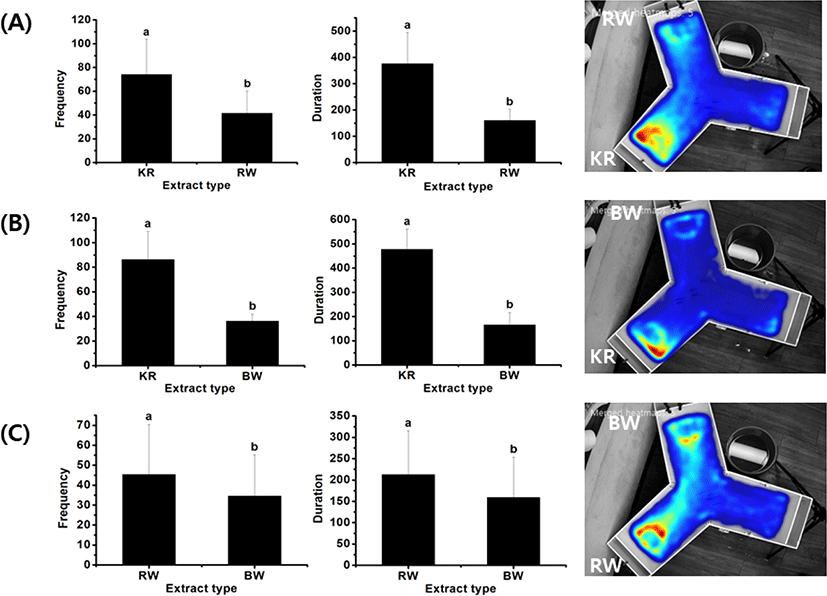
To compare the differences among materials with a moderate level of preference, further analysis was carried out to directly compare between two materials, including krill vs. rock worm, krill vs. bait worm, and rock worm vs. bait worm. The frequency and duration of attraction by rock bream to those pairs of material cues are displayed in Fig. 5 along with their representative heat maps. The results showed that the respective frequency (74 ± 29.8 and 86.3 ± 22.9) and duration (375.6 ± 118.8 and 477.1 ± 84) of the attraction to krill in both experiments were significantly higher than those to rock worm (frequency 41.5 ± 18.7, duration 160.2 ± 42.6) and bait worm (frequency 36.2 ± 5.5, duration 166.1 ± 50.7). The frequency (45.3 ± 25.2) and duration (212.9 ± 102.5) values reflecting rock bream attraction toward rock worm were higher than those toward bait worm (frequency 34.6 ± 20.6, duration 159.1 ± 94.2) (p < 0.05). Overall, these results indicate that the rock bream’s preference toward the test materials was in the following order: fish feed >> sea urchin > krill > rock worm > bait worm >> barley.
Discussion
A behavioral response analysis of fish is one of the simplest and most efficient ways to assess the attraction ability of chemical and visual signals to elicit foraging, courtship, schooling, attracting-evading, or defensive behaviors (Firestein, 2001; Kermen et al., 2013; Mas-Muñoz et al., 2011). Chemical cues play a major role in stimulating food-searching behavior to locate the source of an odorant (Atema, 1980; Vabø et al., 2004). A range of low molecular weight substances, such as amino acids, peptides, terpenoids, nucleotides, nucleosides, fatty acids, bile acids, organic acids, sugars, and other chemicals (Braubach et al., 2011; Carr & Derby, 1986; Elkins et al., 2009; Goh & Tamura, 1980; Johnsen & Adams, 1986) derived from metabolites or chemical stimuli of prey organisms attract fish (Arimoto, 1997; Little & Brewer, 2001; Løkkeborg et al., 2014; Vabø et al., 2004). Apart from these chemical cues, visual cues attract fish toward bait, of which the shape, size, color, and chemical composition are key factors for attracting fish (Alós et al., 2009), which affects the yield and type of fish caught (Broadhurst & Hazin, 2001; Lowry et al., 2006; Smith, 2002; Woll et al., 2001). Therefore, selecting bait with a strong attraction efficacy based on food and feeding habits is important for promoting target fish catchability (Umar et al., 2018).
Several species, such as worms and krill, are popular fishing bait and feed components (Suresh et al., 2011). Various efforts have been focused on developing user and environmentally friendly bait alternatives that can be purchased at a low cost. To be successfully developed as a bait alternative, the target substance should contain at least the threshold level of the chemical cue needed for attracting fish. To identify such a material, we developed a video-based system to screen materials that elicit an attractive response in fish. The reliability of the system was tested with rock bream and two food items—commercial fish feed and barley kernels, which exhibited the highest and lowest attraction efficacy, respectively. A clear preference toward the feed relative to the barley indicated that the system can be applied to identify materials that attract fish. The analysis of rock bream movements in response to the materials offered indicated preference for the materials in the following order: commercial feed > sea urchin > rock worm > bait worm > krill > barley kernel. Commercial feed might have been most strongly preferred due to the ingredient composition of the fish meal (Suresh et al., 2011), although we cannot exclude the effect of farmed fish being adapted to commercial feed. It might have also originated from higher soluble component levels in the feed compared to those in the other materials, which may contain higher muscle or cuticle contents that are not easily soluble (e.g., myofibrillar, alkali-soluble, or stromal proteins). A stronger preference toward sea urchin compared to common bait items (rock worm, bait worm, and krill) could be due to the fact that rock bream typically eat benthic mollusks and crustaceans (i.e., hard-shelled snails and barnacles). The gonads of some sea urchins, which have a distinctive aroma and taste, are used as food and nutraceuticals in some countries (Qin et al., 2011; Siliani et al., 2016; Zhao et al., 2018). Despite their usefulness, sea urchins that overgraze seaweed areas in large numbers can threaten the marine forest environment, by indirectly affecting other marine fauna through their foraging impacts. To protect the marine environment and algal biodiversity, controlling the number of sea urchins that invade seaweed areas is a large challenge in marine production. Thus, the sea urchin population should be controlled from an ecological perspective and the biomass collected consequently should be used effectively. Our study showed that rock bream was more attracted to sea urchin extract compared to those of worms and krill, which have been popularly used as fishing bait. Given that a large amount of sea urchin biomass is expected to be accumulated with control efforts, our findings suggest that sea urchins could be used as a bait alternative. This would be an innovative solution for using up large amounts of sea urchin biomass in an environmentally friendly manner. Additionally, a video tracking system was successfully applied to monitor the movements of rock bream, and this application can be extended to other aquaculture species. Specifically, the system can be successfully applied to identify chemical cues that attract or repel target organisms.
