SHORT COMMUNICATION
Early overcounting in otoliths: a case study of age and growth for gindai (Pristipomoides zonatus) using bomb 14C dating
Allen H. Andrews
1,*
, Taylor R. Scofield
1
1Department of Oceanography, University of Hawaii at Manoa, 1000 Pope Road, Honolulu, Hawaii 96822, HI, USA
Copyright © 2021 The Korean Society of Fisheries and Aquatic Science. This is an Open-Access article distributed under the terms of the
Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits
unrestricted non-commercial use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Received: Oct 28, 2020; Revised: Dec 29, 2020; Accepted: Dec 31, 2020
Published Online: Jan 31, 2021
Abstract
Gindai (Pristipomoides zonatus) is one of six snappers in a management complex called the Deep 7 of the Hawaiian Islands. Little is known about its life history and a preliminary analysis of otolith thin sections indicated the species may exhibit moderate growth with a lifespan approaching 40 years. Preliminary age estimates from the previous study were reinvestigated using the same otolith sections in an attempt to validate those ages with bomb radiocarbon (14C) dating. From the misalignment of birth years for the otolith 14C measurements with regional references — the post-peak bomb 14C decline period — it was concluded that previous ages were inflated from overcounting of the earliest growth zone structure in otolith sections. The oldest gindai was re-aged to 26 years once the age reading was adjusted for early overcounting, 13 years younger than the original estimate of 39 years for this fish. In general, the earliest otolith growth of gindai was massive and complicated by numerous subannual checks. The approach of lumping the early growth structures was supported by the alignment of 14C measurements from otolith core material (first year of growth). The result was greater consistency of calculated birthdates with the 14C decline reference, along with minor offsets that may indicate age estimation was imprecise by a few years for some individuals. The revised von Bertalanffy growth function applied to the validated age-at-length estimates revealed more rapid growth (k = 0.378 cf. 0.113) and a lifespan of approximately 30 years. The findings presented here are a case study of how the bomb 14C decline period can be used as a tool in the refinement of age reading protocols.
Keywords: Otolith; Radiocarbon; Lifespan; Validation; Age Estimation
Introduction
Gindai (Pristipomoides zonatus) is one of six snappers in a management complex called the Deep 7 of the Hawaiian Islands. This species does not comprise a large proportion (1%–2% by weight) of the Deep 7 catch in Hawaii (Langseth et al., 2018), but its importance has increased over the recorded history of this fishery (DeMartini, 2019). The age and growth of this species was first studied in 2013 as a summer project by a University of Hawaii student, in which both otolith sections and gonad samples were worked up as a preliminary life history study (Scofield, 2013). The findings of the study indicated the species could be moderately slow growing (k = 0.113) with a lifespan approaching 40 years. However, these findings were based strictly on estimates of age from growth zone counting that had not been validated. Age-validated life history parameters are essential in understanding resource resilience and in implementing proper management strategies (Cailliet & Andrews, 2008), especially in data-poor fisheries (Newman et al., 2017), like the Deep 7.
A method called bomb radiocarbon (14C) dating can directly assess the accuracy of fish age estimates from otoliths and has evolved considerably over the last 28 years (Andrews et al., 2019b; Kalish, 1993). There is a long history of success with this approach where it has been used to validate or invalidate purported annual growth zones (Andrews et al., 2013; Campana, 1999; Kalish, 1995). The use of this method to age marine organisms relies on a time-specific signal — an observable increase in 14C created by thermonuclear testing in the 1950s and 1960s — and its diffusion to the marine environment (Broecker & Peng, 1982; Druffel et al., 2016; Grottoli & Eakin, 2007). This approach has become an effective tool in providing an age-validated basis for the life history parameters of numerous marine fishes around the world (e.g. Allen & Andrews, 2012; Andrews & Kerr, 2015; Andrews et al., 2005, 2018b; Campana, 1997; Horn et al., 2012; Kalish 2001; Kerr et al., 2004; Vitale et al., 2016) and has been especially effective for species that live in tropical waters (e.g. Andrews et al., 2011a, 2011b, 2013, 2016a, 2020a; Baker & Wilson, 2001; Barnett et al., 2018; Cook et al., 2009; Passerotti et al., 2014) because of increased exposure of the ocean-surface mixed layer to the atmosphere (Andrews et al., 2016b; Druffel et al., 2016). A case study of the Hawaiian pink snapper or opakapaka (P. filamentosus) — also a member of the Deep 7 bottomfish complex and a congener of gindai — was the foundational application of bomb 14C dating to fishes of the Hawaiian Islands (Andrews et al., 2012). This age validation work has continued with other fishes of Hawaii (Andrews et al., 2016a, 2019a, 2020a; DeMartini et al., 2018; Nichols 2019), and has most recently been expanded successfully to use of the post-peak bomb 14C decline period — typically more recent than the 1980s (Andrews et al., 2013, 2018a, 2020; Barnett et al., 2018; Ishihara et al., 2017).
The aim of the current study was to assess the validity of a preliminary life history study of gindai by reinvestigating the age reading to improve the estimates with bomb 14C dating. To do this, a series of youngest to oldest individuals from the original study by Scofield (2013) were selected for bomb 14C analyses. Age estimates from the original study were coupled with measured 14C values from otolith core material (birth year) for a direct comparison of calculated birth years to a coral and otolith 14C reference chronology (Andrews et al., 2016b). This approach can provide a validated basis for estimating the age and growth for gindai — similar to what has now been established for other members of the Deep 7 (Andrews et al., 2012, 2019a, 2020a; Nadon et al., 2020; Nichols, 2019) — with guidance on making adjustments to the age reading of otoliths.
Methods
A set of specimens from the Scofield (2013) study were used in the current study to reassess estimated age using bomb 14C dating (Supplementary Material 1). These otoliths were previously sectioned and investigated for estimates of age using transmitted light (Table 1). The otoliths were from collections made throughout the Hawaiian Archipelago during the years 2007 to 2011. Otoliths were selected across sizes for the 14C analyses for ages and otolith masses that progressed from the youngest and least massive to the oldest and most massive specimens (n = 39; Table 1).
Table 1.
Age estimates for all gindai (Pristipomoides zonatus) otoliths that were selected from the original Scofield (2013) study with region (main and Northwestern Hawaiian Islands [MHI, NWHI]), fish length (fork length), sex, and mean otolith mass. Original age is unvalidated from Scofield (2013) and revised age was from reinvestigation of otolith sections from birthdate misalignments to the regional 14C references
|
Region |
Specimen ID |
Length (FL cm) |
Sex |
Otolith mass (g) |
Original age (yr) |
Revised age (yr) |
F14C ± 1SD |
Revised birthdate |
|
NWHI |
PZ 02 |
20.7 |
M |
0.149 |
2 |
1 |
1.0634 ± 0.0028 |
2007.46 |
|
PZ 16 |
30.7 |
M |
0.195 |
8 |
2 |
1.0667 ± 0.0039 |
2006.47 |
|
PZ 21 |
32.8 |
F |
0.298 |
9 |
3 |
1.0676 ± 0.0039 |
2005.47 |
|
PZ 80 |
35.8 |
F |
0.427 |
17 |
12 |
1.1054 ± 0.0030 |
1995.47 |
|
PZ 81 |
36.5 |
F |
0.389 |
14 |
7 |
1.0898 ± 0.0032 |
2000.47 |
|
PZ 82 |
36.7 |
F |
0.515 |
27 |
10 |
1.1221 ± 0.0034 |
1997.47 |
|
PZ 62 |
39.5 |
M |
0.391 |
15 |
8 |
1.0830 ± 0.0032 |
2000.47 |
|
PZ 87 |
41.2 |
F |
0.609 |
19 |
15 |
1.1245 ± 0.0025 |
1992.47 |
|
PZ 66 |
41.4 |
F |
0.523 |
19 |
7 |
1.0834 ± 0.0033 |
2000.70 |
|
PZ 89 |
41.6 |
M |
0.502 |
17 |
12 |
1.1013 ± 0.0044 |
1995.49 |
|
PZ 68 |
42.0 |
M |
0.575 |
19 |
11 |
1.1124 ± 0.0035 |
1997.47 |
|
PZ 90 |
43.2 |
F |
0.785 |
39 |
26 |
1.1572 ± 0.0030 |
1980.47 |
|
PZ 35 |
43.4 |
F |
0.741 |
26 |
17 |
1.1298 ± 0.0036 |
1990.70 |
|
PZ 72 |
43.8 |
F |
0.591 |
21 |
12 |
1.1043 ± 0.0037 |
1995.70 |
|
PZ 74 |
44.2 |
M |
0.674 |
20 |
16 |
1.1182 ± 0.0037 |
1991.80 |
|
PZ 38 |
44.6 |
F |
0.866 |
28 |
23 |
1.1442 ± 0.0035 |
1984.70 |
|
PZ 39 |
46.5 |
M |
0.679 |
19 |
12 |
1.0990 ± 0.0032 |
1995.80 |
|
PZ 40 |
48.2 |
F |
0.815 |
32 |
22 |
1.1404 ± 0.0029 |
1985.49 |
|
MHI |
PZ 01 |
15.9 |
F |
0.091 |
1 |
0.5 |
1.0579 ± 0.0028 |
2008.41 |
|
PZ 03 |
21.0 |
F |
0.138 |
3 |
1 |
1.0673 ± 0.0024 |
2007.90 |
|
PZ 05 |
23.8 |
F |
0.152 |
3 |
1 |
1.0650 ± 0.0024 |
2008.32 |
|
PZ 11 |
29.0 |
M |
0.206 |
5 |
1 |
1.0617 ± 0.0029 |
2008.32 |
|
PZ 12 |
29.7 |
F |
0.241 |
7 |
2 |
1.0621 ± 0.0025 |
2009.69 |
|
PZ 14 |
30.4 |
F |
0.199 |
6 |
1 |
1.0626 ± 0.0023 |
2008.32 |
|
PZ 20 |
32.5 |
M |
0.242 |
7 |
3 |
1.0574 ± 0.0040 |
2008.68 |
|
PZ 48 |
34.0 |
M |
0.283 |
7 |
3 |
1.0638 ± 0.0028 |
2006.32 |
|
PZ 50 |
34.0 |
F |
0.305 |
12 |
3 |
1.0707 ± 0.0029 |
2006.33 |
|
PZ 22 |
34.0 |
F |
0.317 |
9 |
3 |
1.0669 ± 0.0038 |
2006.33 |
|
PZ 52 |
34.5 |
F |
0.278 |
9 |
3 |
1.0737 ± 0.0027 |
2006.31 |
|
PZ 47 |
34.5 |
M |
0.297 |
12 |
3 |
1.0645 ± 0.0025 |
2006.33 |
|
PZ 55 |
36.0 |
M |
0.309 |
11 |
3 |
1.0624 ± 0.0027 |
2006.33 |
|
PZ 59 |
37.2 |
F |
0.313 |
11 |
4 |
1.0647 ± 0.0026 |
2005.32 |
|
PZ 60 |
38.2 |
F |
0.383 |
16 |
8 |
1.0760 ± 0.0030 |
2001.31 |
|
PZ 65 |
41.2 |
F |
0.650 |
21 |
16 |
1.1204 ± 0.0031 |
1993.31 |
|
PZ 88 |
41.6 |
M |
0.490 |
17 |
8 |
1.0835 ± 0.0027 |
2003.69 |
|
PZ 32 |
42.0 |
F |
0.503 |
18 |
9 |
1.1016 ± 0.0032 |
1999.91 |
|
PZ 69 |
42.5 |
M |
0.479 |
16 |
8 |
1.0720 ± 0.0027 |
2001.31 |
|
PZ 71 |
43.0 |
F |
0.500 |
22 |
15 |
1.1167 ± 0.0031 |
1995.64 |
|
PZ 36 |
43.5 |
M |
0.503 |
15 |
9 |
1.0884 ± 0.0035 |
2002.69 |
Download Excel Table
Bomb radiocarbon dating
Each of the selected otoliths was analyzed for 14C by extracting core material (within the first year of growth), measuring 14C levels in the carbonate sample via accelerator mass spectrometry (AMS), and comparing the measured 14C values and calculated birthdates to regional 14C references. Otolith extractions were performed using a New Wave micromilling machine (Elemental Scientific Lasers, LLC, Bozeman, MT, USA) and a 0.5 mm bur (Brasseler, Savannah, GA, USA). Because the otolith sections were thick (~0.5-0.7 mm), coupled with a massive first year of growth, enough material was available for 14C analysis by extracting the carbonate sample directly from the mounted, thin-sectioned specimen. The mounted glass slide was secured to the milling baseplate with warmed parafilm. The extraction was performed on the micromill using a path length of 2.5 mm at a depth of 0.2 mm in two consecutive passes for a total extraction mass of 0.7–0.8 mg (see Fig. 1 for an example of the core extraction on specimen PZ 90).
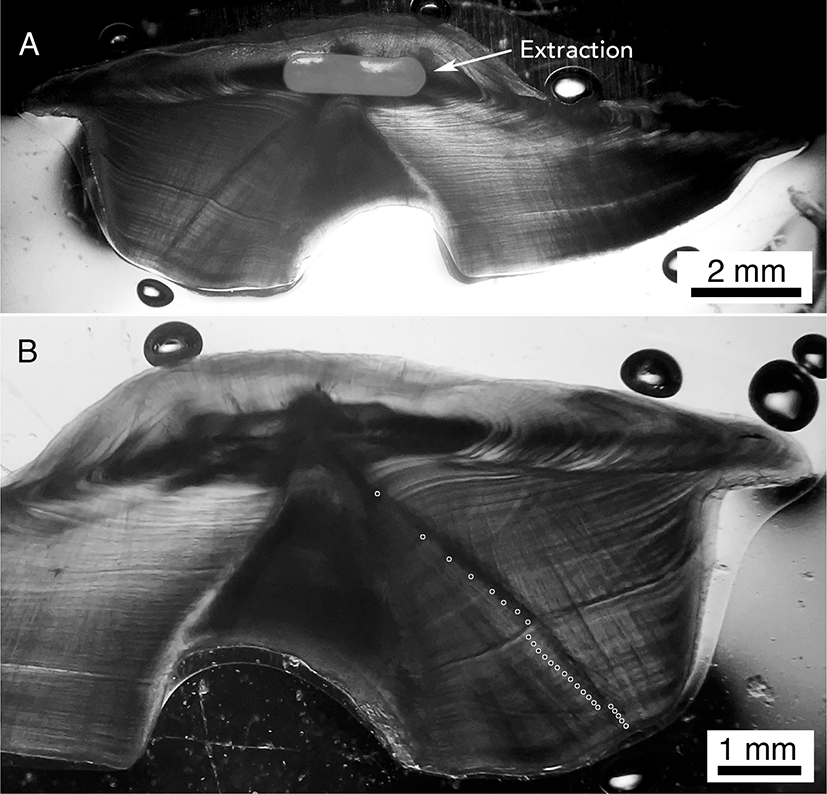
Fig. 1.
Otolith section images from gindai (Pristipomoides zonatus) specimen PZ 90 showing the core extraction (grey shaded area) from the micromill (A) and the revised age-reading scenario (B).
The milled extraction is a trough cut into the original aged otolith section at the core region (2.5 mm long x 0.5 mm wide x 0.4 mm deep) within the first year of growth. The marked section image is the obverse of the same otolith section where lumping of fine growth structure was used for the first 8 years and splitting was used for the remaining growth zones out to 26 years (aged to 25–27 years depending on interpretation). Note that there are numerous ways to count this otolith and that it is possible to attain the original age of 39 years by splitting the earliest growth zones (see Fig. 5 of Scofield (2013) ; Supplementary Material 1). In addition, some growth zone structure that was counted to 26 years is difficult to see because it requires a change in the angle of transmitted light, and may also require panning the eye across the concentric zone structure to either unify or split the area of interest. This age reading protocol was used to age all otoliths that were reinterpreted with support from bomb 14C dating in this study.
Download Original Figure
The extracted otolith samples were submitted as carbonate to the National Ocean Sciences Accelerator Mass Spectrometry Facility (NOSAMS), Woods Hole Oceanographic Institution in Woods Hole, Massachusetts. Radiocarbon measurements were analyzed via routine AMS analyses and reported by NOSAMS as Fraction Modern, the measured deviation of the 14C/12C ratio from Modern. Modern is defined as 95% of the 14C concentration of the National Bureau of Standards Oxalic Acid I standard (SRM 4990B) normalized to δ13C VPDB (–19‰) in 1950 AD (VPDB = Vienna Pee Dee Belemnite geological standard; Coplen, 1996). Radiocarbon results were corrected for isotopic fractionation using δ13C measured concurrently during AMS analysis and are reported here as F14C (Reimer et al., 2004; Stuiver & Polach, 1977).
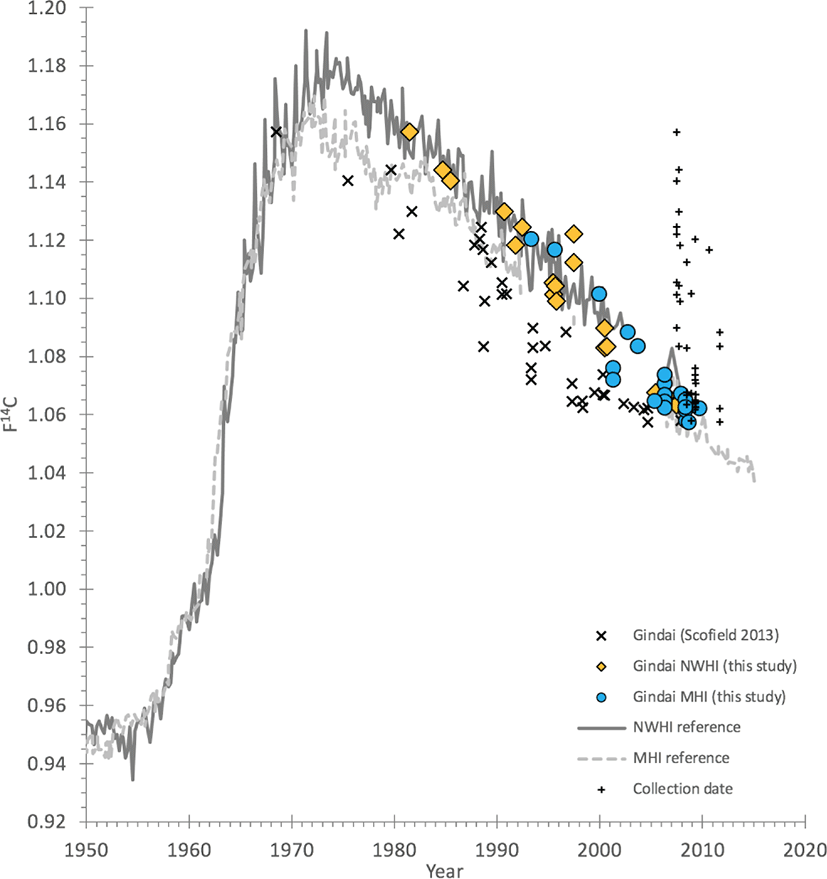
Comparison of measured 14C values at their respective birthdates, and relative to the regional 14C references, provided a baseline for interpreting the validity of age estimates from the Scofield (2013) study (Supplementary Material 1). Alignment or misalignment of the calculated birthdate — determined as the date of collection minus the age estimate — with the years and 14C levels of the post-peak 14C decline (more recent than ~1980) can validate or invalidate the age estimates See(Andrews et al. (2020b) for an example of age scenario elimination using the post-peak bomb 14C decline period). The reference records used in this study were two coral and otolith 14C data sets: 1) Kure Atoll of the Northwestern Hawaiian Islands (NWHI), and 2) Kona of Hawaii Island of the main Hawaiian Islands (MHI; Andrews et al., 2016b; Fig. 2).
Fig. 2.
Bomb 14C plot of the selected series of gindai (Pristipomoides zonatus) otoliths from the early study by Scofield (2013) with revised dates from a reinvestigation section age.
The original estimates of age (X symbol) were used to calculate birth years that are typically well-removed from the expected birth year determined by the regional 14C reference records. Revised age estimates from a combination of 14C alignment and reference-image otolith age-reading led to a stronger alignment of the calculated birth dates with the 14C reference records. Reference records plotted here are from two coral and otolith 14C data sets (Kure Atoll coral and otoliths of the Northwestern Hawaiian Islands (NWHI reference) and Kona of Hawaii Island with otoliths of the main Hawaiian Islands (MHI reference); seeAndrews et al. (2016b) for details).
Download Original Figure
Age estimation and reassessment
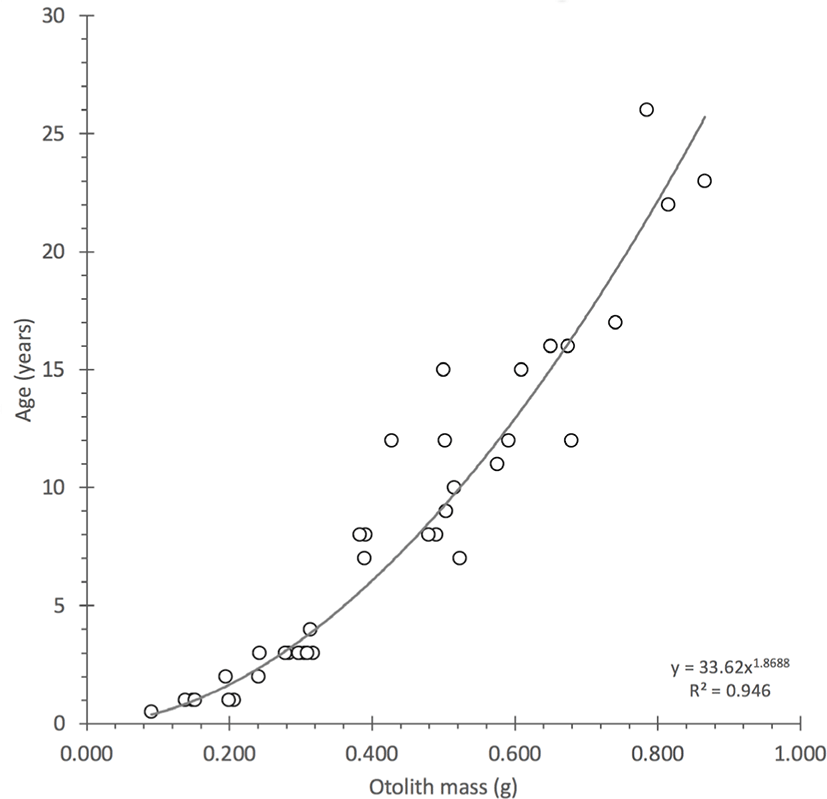
Otoliths were initially prepared and read for estimates of age in a manner that was described in Scofield (2013) and a subset was selected from this study (Table 1; Supplementary Material 1). Because original age estimates led to birth years that were offset from the expected years of formation on the coral reference curves, the ages were recounted using an age reading protocol that is illustrated and enumerated for sample PZ 90 (aged 26 years; Fig. 1). Revised birthdates were calculated from ages counted with the new protocol and were plotted for alignment with the regional bomb 14C decline records. The revised information for age was also correlated with otolith mass because the relation is typically linear or slightly curvilinear and can be used as a tool in the iterative selection of outliers to reassess of estimates of age (Fig. 3). The age-at-length estimates, once verified with alignment to the reference records, were used to generate a revised von Bertalanffy growth function with parameters that can be compared to the Scofield (2013) results.
Fig. 3.
Plot of the validated age estimates with mean otolith mass for gindai (Pristipomoides zonatus) of the Hawaiian Islands indicating a strongly curvilinear relation.
The observation of mass growth indicated half the otolith mass can be accreted in the first 5–10 years of life with the latter otolith growth accreted in the following 15–20 years for this series of specimens.
Download Original Figure
Results and Discussion
Bomb 14C dating revealed that the original age interpretations for gindai from otolith sections were not accurate and that the age reading overcounted growth zones in the earliest otolith growth. The offset observed from the birthdates — calculated from the original age estimates in Scofield (2013) — indicated age was being overestimated and that the overcounting began with the youngest fish. This was evident by the early and consistent offset of the 14C data from the reference chronologies (Fig. 2). Hence, the age reading was restructured from counting of fine increment structure in the earliest otolith growth (See otolith figure in Supplementary Material 1), to grouping or lumping of this fine structure into broader bands of growth (Fig. 1), resulting in reduced age estimates for youngest to oldest fish (Table 1). The grouping of finer growth structure was accomplished by reducing the magnification, and in some instances defocusing the microscope, to allow the eye to see broader more diffuse zones that could be counted (take note of the first 8 years that are marked for specimen PZ 90; Fig. 1). Ultimately, a common early growth structure that could be used to describe more rapid growth was visible among the otolith sections, but it was difficult to quantify. Reference-image otolith age reading — use of what appear to be the best sections for the otolith age reading protocol (Fig. 1; Andrews et al., 2020a; Wakefield et al., 2017) — was employed for sections that needed clarification on how the earliest growth should be counted.
In this study, transmitted light was used on thick sections (~0.5–0.7 mm) where the light diffraction qualities of the otolith matrix were conserved. The reason for this approach is to use the different forms of growth structure (alternating densities due to inclusion of otolin; Campana, 1999) in which angled light transmitted through the otolith can exploit variations in diffraction indices of the otolith matrix — the Leica stereo microscope S8 APO outfitted with the Rotterman ContrastTM transmitted light base (TL4000 RC) is an optimal system for taking advantage of this otolith section artifact to view growth zone structure that is simply not visible with direct transmission (perpendicular to the otolith plane) of light through the otolith section. However, a technique used by Ong et al. (2016) described a method that used a brightfield on thin sectioned otoliths (0.15–0.19 mm) and the growth zone structure was clearly visible. While the older growth zones were fairly well defined for gindai in thicker sections, the problems with grouping early zones may not be solved with ultra-thin sections. One potential solution was with use of the crenulated and knob-like structures seen along the dorsal and ventral axes of the thin sections (Fig. 1B) — numerous smaller zones seen in the early growth structure could be lumped based on an association with these structural features. This approach was used in some cases for gindai and the approach was familiar from previous work on other fishes where there were counting problems in the earliest otolith growth (e.g., Andrews et al., 1999, 2005, 2013, 2018b; Kerr et al., 2004).
Once the ages for gindai were reevaluated relative to the bomb 14C decline period and a revised counting scenario was developed, the alignment of the birthdates were in closer agreement with the expected 14C decline dates (Fig. 2). Some remain offset and this may be due to either, 1) unresolved ages that are actually older or younger than estimated by a few years, or 2) greater offsets in 14C due to regional variations that have not been accounted for — each 14C reference record is from one end of the Hawaiian Archipelago to the other and the gyre waters of the North Pacific have been demonstrated to have a range of water sources that can be 14C-depleted for various reasons (Andrews et al., 2016b; Kumamoto et al., 2013). Regardless, the revised estimates are much closer to the actual age of the gindai specimens — on the basis of clear misalignment of the preliminary study birthdates (Fig. 2) — than the original estimates provided by Scofield (2013).
Because the revised age estimates from growth zone counting are likely accurate to within a few years based on the alignment of calculated birthdates to the bomb 14C decline (Fig. 2), the values were used to generate a von Bertalanffy growth function from the age-at-length data (Fig. 4). The life history parameters differed greatly from the original study by Scofield (2013) in that growth rate was greater and lifespan was lower. The rapid early growth exhibited by the revised growth curve was considerably different (k = 0.378 cf. 0.113) and closer to estimates made in a preliminary study of the same species off Western Australia (WA; estimated k = 0.26–0.30; Corey Wakefield, Western Australian Fisheries and Marine Research Laboratories, Department of Fisheries, Government of Western Australia; pers. comm. 2019). While the k value presented here is also greater than what was observed in WA, the growth function was based on few individuals and could change with the addition of more aged gindai from Hawaii using the proposed age reading protocol presented here (Fig. 1). Furthermore, the unpublished data from WA is supported with an approach similar to dendrochronology where identifiable growth events were well-correlated in time (e.g., Ong et al., 2016). Longevity is lower than originally estimated by Scofield (2013) and may be closer to 30 years in Hawaii. However, the findings from WA indicate the species may live more than 50 years from the well-defined age reading protocol (Corey Wakefield, Western Australian Fisheries and Marine Research Laboratories, Department of Fisheries, Government of Western Australia; pers. comm. 2019). It must be noted that gindai in Hawaii may not live 50 years because otoliths with a mass approaching 0.9 g (aged to early to mid-20s) were rare in collections across the Hawaiian Islands, assuming otolith mass is a reasonable proxy for age given the strongly curvilinear relationship (Table 1; Fig. 3).
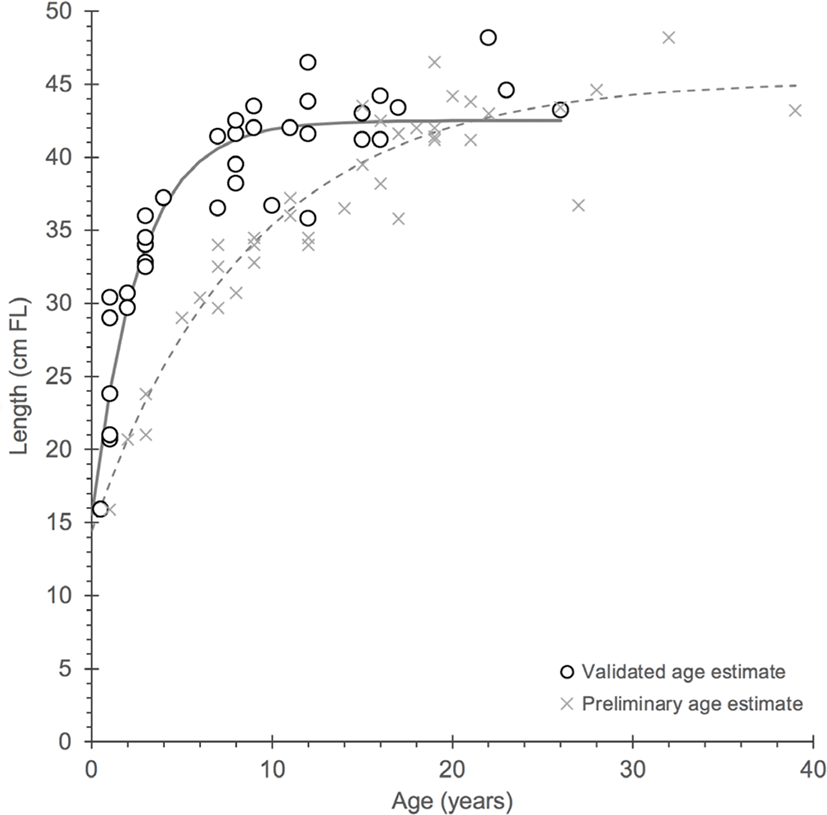
Fig. 4.
Von Bertalanffy growth functions for gindai (Pristipomoides zonatus) in the Hawaiian Islands from the preliminary Scofield (2013) work (X symbol) and the current study (open circles) from which a considerable contrast can be observed between otolith age interpretations and growth parameters.
The early work by Scofield (2013) was based on observations of purportedly annual growth zones that appeared to be well-defined, resulting in a moderate growth rate (k = 0.113) and maximum age approaching 40 years. Reassessed ages that were validated with bomb 14C dating reveal a growth rate that is significantly greater (k = 0.378) with a reduced maximum age approaching 30 years. Growth function parameters for Scofield (2013): L∞ = 45.3 cm FL, k = 0.113 yr–1 and t0 = –3.4 yr and the current study: L∞ = 42.5 cm FL, k = 0.378 yr–1 and t0 = –1.2 yr. These findings also indicate that age at 50% maturity (A50) is earlier than 6 years (the original estimate by Scofield (2013)) and is closer to 2 years (L50 = 29.7 cm FL).
Download Original Figure
Overall, the findings of this study reveal that age can be easily overestimated when validation is not attempted — studies that employ only otolith section age-reading run the risk of establishing invalid life history characteristics (e.g. O’Malley et al., 2019). Growth parameters from Scofield (2013) were believable at the time because a consistent age reading protocol was developed and other regional snapper species were longer lived than previously thought. Onaga (Etelis coruscans), another member of the Deep 7, has a k value of 0.105–0.126 (Andrews et al., 2021), which was consistent with the early gindai study. However, its congener opakapaka (P. filamentosus) — along with ehu (E. carbunculus) — have more rapid growth with k values on the order of 0.20–0.25 (Andrews et al., 2012; Nichols, 2019). It follows that a species often landed with the Deep 7 fishes, the uku or grey snapper (Aprion virescens), exhibits a rapid growth rate (k ≈ 0.3) that is similar to gindai with a validated lifespan from bomb 14C dating of 25 years (DeMartini, 2019; Nadon et al., 2020). Hence, the importance of employing age validation techniques, like the unique utility of the bomb 14C decline period, to authenticate age reading protocols and the estimates of age and growth derived from otolith sections are highlighted here for deep-water snapper in Hawaii and are broadly applicable to tropical marine fishes.
Acknowledgements
Thanks to various regional fishers (Eddie Ebisui, Roy Moribe, Layne Nakagawa, Clay Tam, Bradley Saito) for providing specimens, Jeff Sampaga for otolith extractions, and Meagan Sundberg (Luers) for assistance with collating otoliths from the collections for use in this study. Ryan Nichols was an age reader that corroborated the refined age reading protocol and I am thankful for his participation. Thanks to Robert Humphreys, Edward DeMartini, Cynthia Hunter (University of Hawaii, Department of Biology, Marine Option Program), and the Pacific Islands Fisheries Science Center for project support. This research did not receive any specific funding and the authors declare no conflicts of interest. Specimen collections were made under the jurisdiction of the National Marine Fisheries Service in accordance with the recommended guidelines for use of fishes in research (https://fisheries.org/policy-media/science-guidelines/guidelines-for-the-use-of-fishes-in-research/). Data accessed for this study is available at the public repository of National Centers for Environmental Information (https://www.ncdc.noaa.gov/paleo-search/study/27541) via the Public Access to Research Results (PARR), White House Office of Science and Technology Policy (OSTP) Memorandum Increasing Access to the Results of Federally Funded Scientific Research.
Availability of data and materials
Ethics approval and consent to participate
References
Allen LG, Andrews AH. Bomb radiocarbon dating and estimated longevity of Giant Sea Bass (Stereolepis gigas). Bull South Calif Acad Sci. 2012; 111:1-14


Andrews AH. Giant trevally (Caranx ignobilis) of Hawaiian Islands can live 25 years. Mar Freshw Res. 2020; 71:1367-72


Andrews AH, Kerr LA. Estimates of maximum age for white sharks of the northeastern Pacific Ocean: altered perceptions of vertebral growth shed light on complicated bomb Δ
14C results. Environ Biol Fishes. 2015; 98:971-8


Andrews AH, Cailliet GM, Coale KH. Age and growth of the Pacific grenadier (Coryphaenoides acrolepis) with age estimate validation using an improved radiometric ageing technique. Can J Fish Aquat Sci. 1999; 56:1339-50


Andrews AH, Burton EJ, Kerr LA, Cailliet GM, Coale KH, Lundstrom CC, et al. Bomb radiocarbon and lead-radium disequilibria in otoliths of bocaccio rockfish (Sebastes paucispinis): a determination of age and longevity for a difficult-to-age fish. Mar Freshw Res. 2005; 56:517-28


Andrews AH, Kalish JM, Newman SJ, Johnston JM. Bomb radiocarbon dating of three important reef–fish species using Indo–Pacific ∆
14C chronologies. Mar Freshw Res. 2011a; 62:1259-69


Andrews AH, Natanson LJ, Kerr LA, Burgess GH, Cailliet GM. Bomb radiocarbon and tag-recapture dating of sandbar shark (Carcharhinus plumbeus). Fish Bull. 2011b; 109:454-65.

Andrews AH, DeMartini EE, Brodziak J, Nichols RS, Humphreys. A long-lived life history for a tropical, deepwater snapper (Pristipomoides filamentosus): bomb radiocarbon dating as extensions of daily increment analyses in otoliths. Can J Fish Aquat Sci. 2012; 69:1850-69


Andrews AH, Barnett BH, Allman RJ, Moyer RP, Trowbridge HD. Great longevity of speckled hind (Epinephelus drummondhayi), a deep-water grouper, with novel use of postbomb radiocarbon dating in the Gulf of Mexico. Can J Fish Aquat Sci. 2013; 70:1131-40


Andrews AH, DeMartini EE, Eble JA, Taylor BM, Lou DC, Humphreys RL. Age and growth of bluespine unicornfish (Naso unicornis): a half-century lifespan for a keystone browser, with a novel approach to bomb radiocarbon dating in the Hawaiian Islands. Can J Fish Aquat Sci. 2016a; 73:1575-86


Andrews AH, Siciliano D, Potts DC, DeMartini EE, Covarrubias S. Bomb radiocarbon and the Hawaiian Archipelago: coral, otoliths and seawater. Radiocarbon. 2016b; 58:531-48


Andrews AH, Humphreys RL, Sampaga JD. Blue marlin (Makaira nigricans) longevity estimates confirmed with bomb radiocarbon dating. Can J Fish Aquat Sci. 2018a; 75:17-25


Andrews AH, Smale MJ, Cowley PD, Chang N. Fifty-five-year longevity for the largest member of the family Sparidae: the endemic red steenbras Petrus rupestris from South Africa. Afr J Mar Sci. 2018b; 40:343-53


Andrews AH, DeMartini EE, Brodziak J, Nichols RS, Humphreys RL. Growth, longevity, and age at first maturity and sex change of Hawaiian grouper (Hyporthodus quernus) — input for management and conservation of a large, slow-growing grouper. Can J Fish Aquat Sci. 2019a; 76:1874-84


Andrews AH, Yeman C, Welte C, Hattendorf B, Wacker L, Christl M. Laser ablation accelerator mass spectrometry reveals complete bomb
14C signal in an otolith with confirmation of 60-year longevity for red snapper (Lutjanus campechanus). Mar Freshw Res. 2019b; 70:1768-80


Andrews AH, Brodziak J, DeMartini EE, Cruz E. Long-lived life history for onaga (Etelis coruscans) in the Hawaiian Islands. Mar Freshw Res. 2020a


Andrews AH, Pacicco A, Allman R, Falterman BJ, Lang ET, Golet W. Age validation of yellowfin (Thunnus albacares) and bigeye (Thunnus obesus) tuna of the northwestern Atlantic Ocean. Can J Fish Aquat Sci. 2020b; 77:637-43


Baker MS, Wilson CA. Use of bomb radiocarbon to validate otolith section ages of red snapper Lutjanus campechanus from the northern Gulf of Mexico. Limnol Oceanogr. 2001; 46:1819-24


Barnett BK, Thornton L, Allman R, Chanton JP, Patterson WF. Linear decline in red snapper (Lutjanus campechanus) otolith Δ
14C extends the utility of the bomb radiocarbon chronometer for fish age validation in the Northern Gulf of Mexico. ICES J Mar Sci. 2018; 75:1664-71


Broecker WS, Peng TH. Tracers in the sea. New York, NY: Lamont–Doherty Geological Observatory, Columbia University. 1982.

Cailliet GM, Andrews AH. Age-validated longevity of fishes: its importance for sustainable fisheries.In In: Tsukamoto K, Kawamura T, Takeuchi T, Beard TD, Kaiser MJ, editors.editors Fisheries for global welfare and environment, 5th World Fisheries
Congress. 2008; Yokohama. Japan: p p. 103-20.

Campana SE. Use of radiocarbon from nuclear fallout as a dated marker in the otoliths of haddock Melanogrammus aeglefinus. Mar Ecol Prog Ser. 1997; 150:49-56


Campana SE. Chemistry and composition of otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser. 1999; 188:263-97


Cook M, Fitzhugh GR, Franks JS. Validation of yellowedge grouper, Epinephelus flavolimbatus, age using nuclear bomb-produced radiocarbon. Environ Biol Fishes. 2009; 86:461-72


Coplen TB. New guidelines for reporting stable hydrogen, carbon, and oxygen isotope-ratio data. Geochim Cosmochim Acta. 1996; 60:3359-60


DeMartini EE. Hazards of managing disparate species as a pooled complex: a general problem illustrated by two contrasting examples from Hawaii. Fish Fish. 2019; 20:1246-59


DeMartini EE, Andrews AH, Howard KG, Taylor BM, Lou DC, Donovan MK. Comparative growth, age at maturity and sex change, and longevity of Hawaiian parrotfishes, with bomb radiocarbon validation. Can J Fish Aquat Sci. 2018; 75:580-89


Druffel ERM, Beaupré SR, Ziolkowski LA. Radiocarbon in the oceans.In In: Schuur EAG, Druffel E, Trumbore SE, editors.editors Radiocarbon and climate change: mechanisms, applications and laboratory
techniques. Cham, Switzerland: Springer. 2016; p p. 139-66


Grottoli AG, Eakin CM. A review of modern coral δ
18O and δ
14C proxy records. Earth Sci Rev. 2007; 81:67-91


Horn PL, Neil HL, Paul LJ, McMillan PJ. Age verification, growth and life history of rubyfish Plagiogeneion rubiginosum. N Z J Mar Freshw Res. 2012; 46:353-68


Ishihara T, Abe O, Shimose T, Takeuchi Y, Aires-da-Silva A. Use of post-bomb radiocarbon dating to validate estimated ages of Pacific bluefin tuna, Thunnus orientalis, of the North Pacific Ocean. Fish Res. 2017; 189:35-41


Kalish JM. Pre- and post-bomb radiocarbon in fish otoliths. Earth Planet Sci Lett. 1993; 114:549-54


Kalish JM. Radiocarbon and fish biology.In In: Secor DH, Dean JM, Campana SE, editors.editors Recent developments in fish otolith research. Columbia, SC: University of South Carolina Press. 1995; p p. 537-653.

Kalish JM, Nydal R, Nedreaas KH, Burr GS, Eine GL. A time history of pre- and post-bomb radiocarbon in the Barents Sea derived from Arcto-Norwegian cod otoliths. Radiocarbon. 2001; 43:843-55


Kerr LA, Andrews AH, Frantz BR, Coale KH, Brown TA, Cailliet GM. Radiocarbon in otoliths of yelloweye rockfish (Sebastes ruberrimus): a reference time series for the coastal waters of southeast Alaska. Can J Fish Aquat Sci. 2004; 61:443-51


Kumamoto Y, Murata A, Kawano T, Watanabe S, Fukasawa M. Decadal changes in bomb-produced radiocarbon in the Pacific Ocean from the 1990s to 2000s. Radiocarbon. 2013; 55:1641-50


Langseth B, Syslo J, Yau A, Kapur M, Brodziak J. Stock assessment for the main Hawaiian Islands deep 7 bottomfish complex
in 2018, with catch projections through 2022. Washington, DC: United States Department of Commerce. 2018Report No.: NOAA-TM-NMFS-PIFSC-69.

Nadon MO, Sculley M, Caravalho F. Stock assessment of uku (Aprion virescens) in Hawaii, 2020. Washington, DC: United States Deppartment of Commerce. 2020Report No.: NOAA-TM-NMFS-PIFSC-100.

Newman SJ, Wakefield CB, Williams AJ, O’Malley JM, Taylor BM, Nicol SJ, et al. International workshop on advancing methods to overcome challenges associated with life history and stock assessments of data-poor deep-water snappers and groupers. Mar Policy. 2017; 79:78-3


Nichols RS. Sex-specific growth and longevity of ‘ehu’, Etelis
carbunculus (Family Lutjanidae), within the Hawaiian Archipelago. [M.S. Thesis], Hawaii, HI: University of Hawaii. 2019; p, p. 137.

O’Malley JM, Wakefield CB, Oyafuso ZS, Nichols RS, Taylor B, Williams AJ, et al. Effects of exploitation evident in age-based demography of 2 deepwater snappers, the goldeneye jobfish (Pristipomoides flavipinnis) in the Samoa Archipelago and the goldflag jobfish (P. auricilla) in the Mariana Archipelago. Fish Bull. 2019; 117:322-36


Ong JJL, Rountrey AN, Marriot RJ, Newman SJ, Meeuwig JJ, Meekan MG. Cross-continent comparisons reveal differing environmental drivers of growth of the coral reef fish, Lutjanus bohar. Coral Reefs. 2016; 36:195-206


Passerotti MS, Andrews AH, Carlson JK, Wintner SP, Goldman KJ, Natanson LJ. Maximum age and missing time in the vertebrae of sand tiger shark (Carcharias taurus): validated lifespan from bomb radiocarbon dating in the western North Atlantic and southwestern Indian Oceans. Mar Freshw Res. 2014; 65:674-87


Reimer PJ, Brown TA, Reimer RW. Discussion: reporting and calibration of post-bomb
14C data. Radiocarbon. 2004; 46:1299-304


Scofield T. (2013) Age and maturation of gindai (Pristipomoides zonatus), a Hawaiian
bottomfish. Honolulu, HI: University of Hawaii at Manoa. 2013Summer Student Project (BIOL 499) [Attached to this publication as Supplementary Material 1].

Stuiver M, Polach HA. Discussion: reporting of
14C data. Radiocarbon. 1997; 19:355-63


Vitale S, Andrews AH, Rizzo P, Gancitano S, Fiorentino F. Twenty-five-year longevity for European hake (Merluccius merluccius) from novel use of bomb radiocarbon dating in the Mediterranean Sea. Mar Freshw Res. 2016; 67:1077-80


Wakefield CB, O’Malley JM, Williams AJ, Taylor BM, Nichols RS, Halafihi T, et al. Ageing bias and precision for deep-water snappers: evaluating nascent otolith preparation methods using novel multivariate comparisons among readers and growth parameter estimates. ICES J Mar Sci. 2017; 74:193-203


Williams AJ, Newman SJ, Wakefield CB, Bunel M, Halafihi T, Kaltavara J, et al. Evaluating the performance of otolith morphometrics in deriving age compositions and mortality rates for assessment of data-poor tropical fisheries. ICES J Mar Sci. 2015; 72:2098-109