Introduction
Since 2000, the abalone farming industry in Korea has soared due to an increase in fishing licenses issued and cage facilities; in 2019, 20 059 tons of abalone were produced at a value of approximately KRW 610.3 billion (KNSO, 2019). Most abalone farms are in Jeollanam-do and account for 98% of the total production in Korea (Shin et al., 2017).
Abalone farming uses cages and is greatly affected by natural changes (e.g., climatic and other disasters). Due to recent climate change factors, the number of epibionts in cage facilities and on abalone shells is increasing as the water temperature in abalone farming areas increases, and it has been reported that this causes problems such as impaired growth, increased mortality, and a decrease in product value (Won et al., 2013). Epibionts growing on abalone shells make feeding and other farming activities difficult. Another threat is that breeding holes are blocked by the epibionts. The cost of removing the epibionts is KRW 20 billion per year. However, there is a lack of studies on the effects of and possible control measures for epibionts.
In this study, the growth of abalone according to the proportion of epibionts present was investigated for abalone cultured in Goheung, Jeollanam-do, where high water temperatures and attachment problems have been steadily increasing.
Materials and Methods
In this study, Haliotis discus hannai raised it in a cage in Goheung (Geumsan-myeon, Goheung-gun), Jeollanam-do at a standard density of less than 4 cm (4 cm, 2,000 abalone), and then divided it into two cages and raised it for eight months (May-December 2020). In one cage (epibiont removal, ER), the epibionts were removed once, in August, and in the other cage (non-epibiont removal, NER) the abalone were raised for eight months without removing the epibionts. Forty abalone were collected every month and the shells of these individuals were measured for shell height (SH, mm), shell length (SL, mm), total weight (TW, g), and body weight (BW, g); they were then and analyzed for the condition index (CI).
Each month, the species of epibiont on the abalone shells were classified based on their morphological characteristics and the proportion (%) of the three dominant species among them was analyzed. In addition, the weight of the attachment was dropped using an air chipper (ECO20-L, Ecotool, Smith, Seoul, Korea), and the weight was measured:
Results
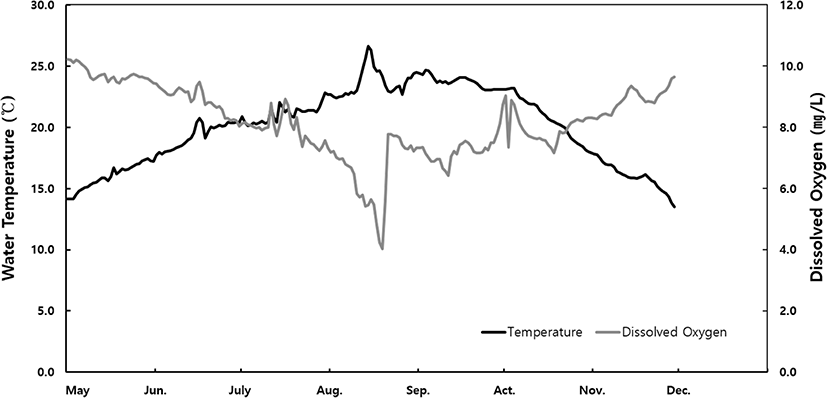
Daily variations in water temperature and dissolved oxygen from May to December 2020 are shown in Fig. 1. In August, the water temperature was highest at a range from 13.5°C to 26.6°C and dissolved oxygen was lowest at a range from 4.0 to 10.2 mg/L; however, these were within the ranges for normal abalone habitat.
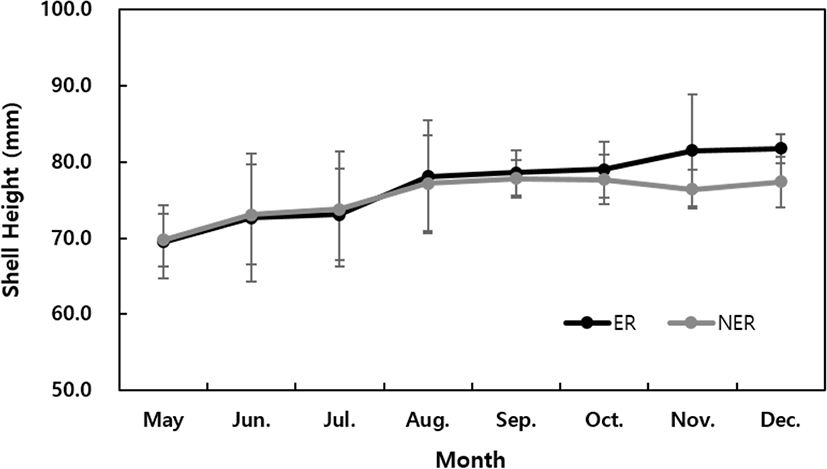
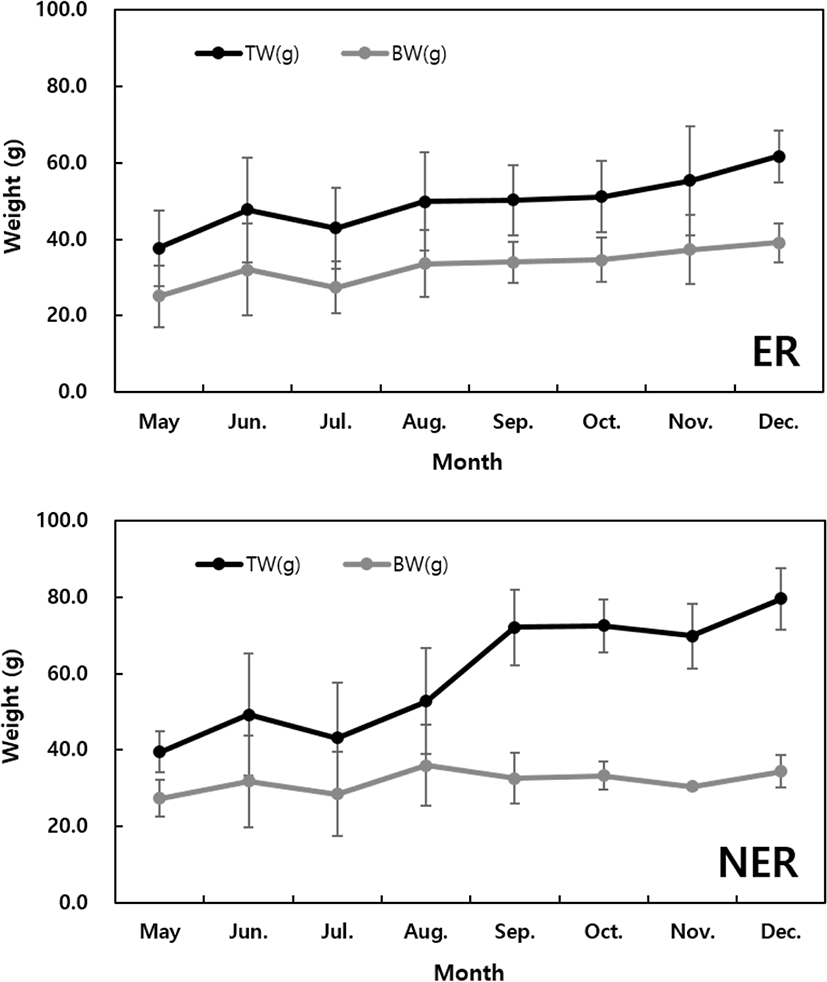
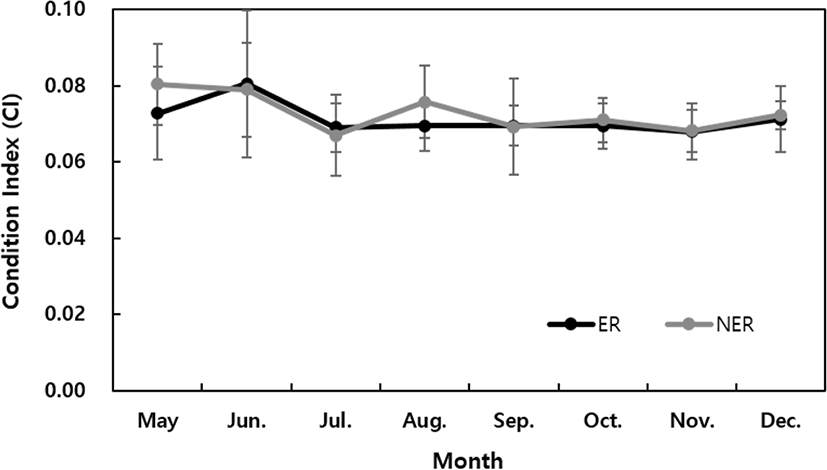
The SH (mm) of the abalone grew by 117.7% (81.8 ± 1.9 mm) in the ER and 111% (77.4 ± 3.3 mm) in the NER compared to the initial measurements (69.61 mm) (Fig. 2). During the study period, the TW and BW increased significantly and steadily in the ER condition, whereas after August the TW increased sharply in the NER, while the BW tended to decrease (Fig. 3). No significant difference was observed in the CI between the ER and NER (Fig. 4).
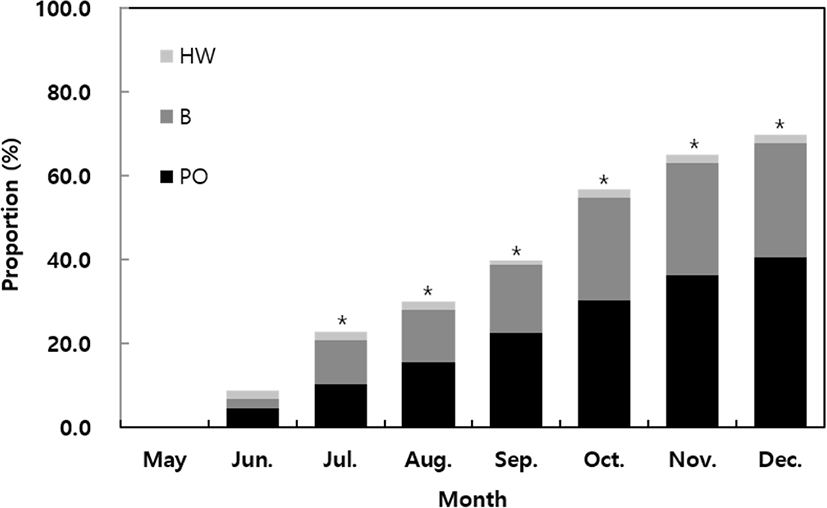
Among the epibionts on the surface of abalone shells, the most dominant species were Pacific oysters (Crassostrea gigas), barnacles (Chthalamus spp.), and hydroid warms (Table 1). Analysis of the monthly proportion of epibionts showed that this increased significantly from July until it reached a value of 69.9% in December. Oysters accounted for the highest percentage of attachments (40.6%), followed by barnacles (27.3%); the ratio of oysters and barnacles was similar until August but increased sharply from September (Fig. 5).
| Epibionts | Occurrence (%) |
|---|---|
| Total | 69.9 |
| Pacific oyster (Crassostrea gigas) | 40.6 |
| Barnacles (Chthalamus spp.) | 27.3 |
| Hydroid warms | 2.0 |
Discussion
In Korea, abalone is mainly grown in cages; therefore, their survival and growth are greatly influenced by exogenous factors (Kim et al., 2005; Lee, 2014; Park et al., 2013; Shin et al., 2011). Abalone growth is closely related to water temperature (Britz et al., 1997; Cho & Cho, 2009; Sakai, 1962), density (Capinpin et al., 1999; Kim et al., 2013a; Kim et al., 2014; Yoon et al., 2014), and diets (Kim et al., 2003; Kim et al., 2013b; Lee et al., 1999).
In this study, the abalone were raised at a standard density and the factors affecting their growth-including the equitable supply and management of Laminaria japonica for food–were minimized.
Recently, the value of abalone has been decreasing due to the presence of epibionts, and the cost of removing them is increasing every year. Abalone shell attachments can be both internal and external; perforation by Polydora, which are internal attachments, do not cause death; however, they have been reported to reduce abalone growth and weight and increase stress (Bower et al., 1994; Kent, 1979; Won et al., 2013). However, there is a lack of studies on the effects of and control measures for organisms that attach externally on abalone.
In addition, this study revealed that the proportion of epibionts increased after September; barnacles, in particular, increased after June. The spawning period of oysters and barnacles were late August and early June, respectively (Kim et al., 2009).
The proportion of epibionts differed according to the growth of the attachments. SHs, TWs, and BWs increased significantly from September in the ER group; however, the SHs did not increase in the NER group and the BWs tended to decrease. These findings suggest that epibionts affect abalone growth indirectly.
Although epibionts have adverse effects on ship hulls, fish cages, seaside facilities, and aquaculture in various forms, domestic research on this topic is insufficient (Park, 1980; Shim & Jung, 1987). Therefore, it seems that more control studies using the spawning time and attraction substrate of the attachments, as well as a study on the effect of these on abalone, are necessary.
