Introduction
The file fishes (Monacanthidae) mainly inhabit shallow coastal reefs and algal beds depths of 10–200 m (Hutchins, 2001; Nelson et al., 2016). The body of the file fish is laterally compressed, and their skins are covered with small spines. In addition, they have two separated dorsal fins, the first dorsal fin usually with two spines, while the second usually smaller or absent (Nelson, 1994). Four species of Brachaluteres are reported worldwide (Froese & Pauly, 2021), with one species recorded in Japanese waters (Hayashi & Hagiwara, 2013), but no occurrence reported in Korea waters. The genus Brachaluteres is characterized by no protruded pelvic bone lacking spine at its end, dorsal and anal rays short and low with all less than 30 rays, and subcircular body (Hutchins & Swainston, 1985).
The first record of B. ulvarum has been reported as a new species in a bay around Misaki Japanese waters in 1902 (Jordan & Fowler, 1902), but there has been a lack of data on their additional occurrence and life history. Only a study of their spawning behavior was conducted by Akagawa et al. (1995). This species is distributed in relatively narrow area along the southern coast of Japan and listed in the category of least concern (LC) in the list of IUCN (Matsuura et al., 2019).
In this study, we report the first occurrence of B. ulvarum in Korean waters based on one specimen collected in SCUBA diving from the Supseom, Jeju-do Island. The identification of the species was grounded on the morphological characteristics, and records of its mitochondrial cytochrome c oxidase subunit I (mt COI) sequences.
Materials and Methods
One specimen of B. ulvarum was collected from Supseom (33°13’47.50” N, 126°35’45.89” E) in the southern coast of Jeju-do Island, Korea, via SCUBA diving on 6 March 2021 (Fig. 1). In the laboratory, measurements and meristic counts were recorded following the method of Hutchins (1977). The sizes of body parts were measured to the nearest 0.1 mm using digital Vernier calipers with add of microscope. The specimen was then preserved in 5% formalin for 24 h and later transferred to 70% ethanol for depositing at the Marine Biodiversity Institute of Korea (MABIK).
To compare molecular data, total genomic DNA was extracted from the muscle tissue using 10% Chelex resin (Bio-Rad, Hercules, CA, USA). A portion of the mitochondrial COI gene was amplified using universal primers (Ward et al., 2005). PCR was performed in a 30 μL reaction tube containing 1 μL genomic DNA, 2 μL 10× PCR buffer, 2 μL 2.5 mM dNTP, 1 μL of each primer, 0.1 μL Ex-Taq DNA polymerase, and 22.9 μL sterile distilled H2O, using a thermal cycler (MJmini PTC-1148, Bio-Rad). The PCR profile consisted of initial denaturation at 95°C for 5 min, followed by 34 cycles of denaturation at 95°C for 1 min, annealing at 50°C, extension at 72°C for 1 min, and a final extension at 72°C for 5 min. PCR products were purified using ExoSAP-IT (United States Biochemical, Cleveland, OH, USA) and sequenced using an ABI PRISM BigDye Terminator v3.1 Ready Reaction cycle sequencing kit (Applied Biosystems, Waltham, MA, USA) on an ABI 3730xl DNA analyzer (Applied Biosystems). We compared our molecular data with those of the mtDNA COI sequences from other Brachaluteres species and one outgroup, Paraluteres prionurus (KP194657), download from the NCBI GenBank (National Center for Biotechnology Information, www.ncbi.nlm.nih.gov). Sequences were aligned using ClustalW (Thompson et al., 1994) in BioEdit, version 7 (Hall, 1999). The genetic divergences were calculated using the Kimura 2-parameter (K2P) (Kimura, 1980) model with Mega 6 (Tamura et al., 2013). Phylogenetic trees were constructed using the neighbor-joining method (Saitou & Nei, 1987) in Mega 6 (Tamura et al., 2013), with confidence assessed based on 1,000 bootstrap replications.
Results and Discussion
BrachaluteresBleeker (1865): 100 (type species: Aleuterius trossulus)
Body slightly compressed, subcircular; gill opening placed above pectoral fin base; no protruded pelvic bone lacking spine at its end; dorsal ray and anal ray short and low with all less than 30 soft rays (Hutchins & Swainston, 1985).
Brachaluteres ulvarumJordan & Fowler (1902): 271 (type locality: Misaki, Japan); Matsuura et al. (1984): 361 (Japan); Akagawa et al. (1995): 397 (Japan); Hayashi (2002): 1404 (Japan).
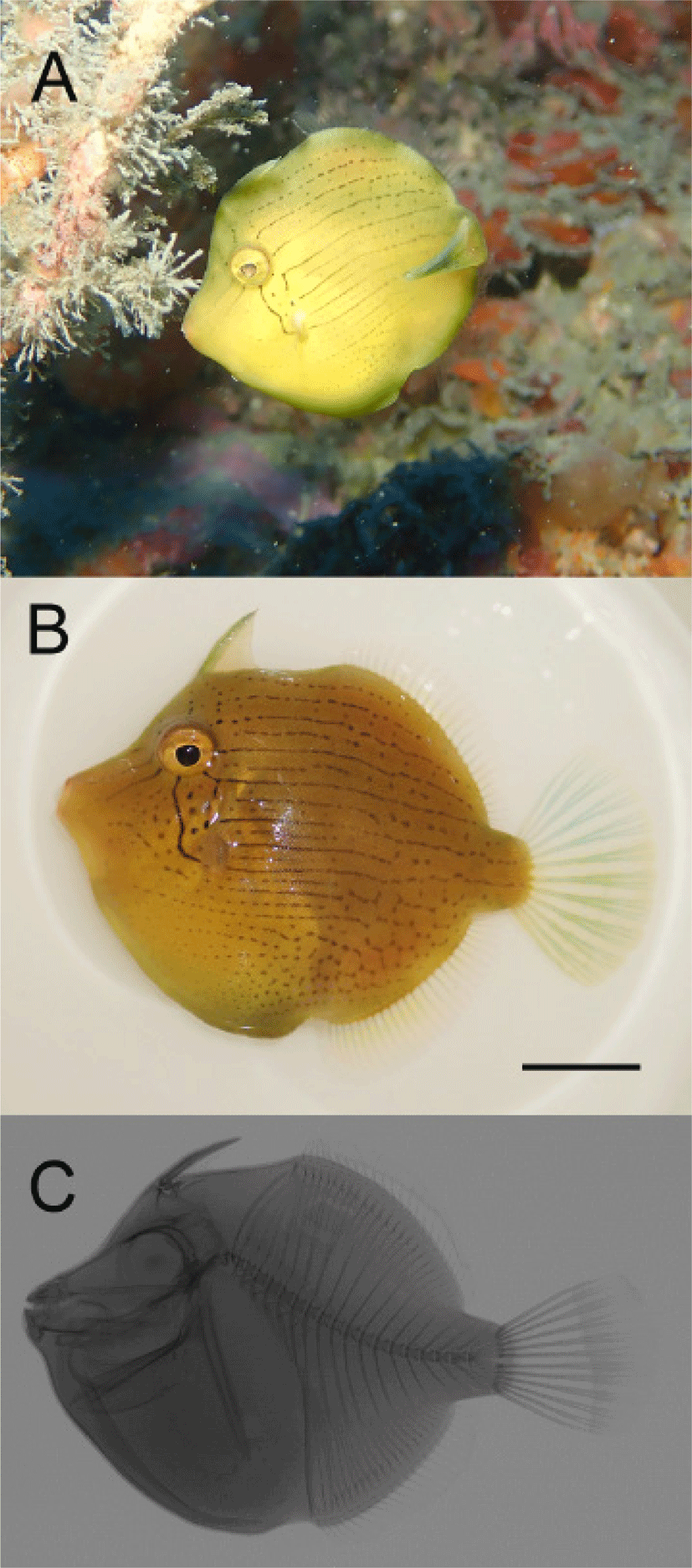
Brachaluteres ulvarum (MABIK PI00049731) 39.4mm standard length (SL) Supseom, Jeju-do Island, Korea, 33°13’47.50” N, 126°35’45.89” E, rocky substrate, 12 m, coll. S.H. Myoung (SCUBA diving), hand net, 6 March 2021.
Body counts, measurements, and proportions of body parts against to SL are shown in Table 1. Body deep, compressed, and generally round (Fig. 2). Head short and deep. Mouth small and terminal. Belly rounded downwards, without protruding spine. Origin of first dorsal spine slightly behind center of the eye, and gill slit the behind of eye. Origin of pectoral fin just below gill slit. Pectoral fin short and its end round. First dorsal spine slender, end of spine connected by a thin membrane. Second dorsal spine very small, located behind the first dorsal spine (Fig. 2C). Heights of second dorsal fin and anal fin short. Origin of these fins almost in the middle of body with origin of anal fin slightly behind, and both ends reach caudal peduncle part. Caudal fin big and its end round.
| Morphological characters | Present specimen | Hutchins & Swainston (1985) | Jordan & Fowler (1902) |
|---|---|---|---|
| No. of specimen | 1 | 5 | 1 |
| Standard length (mm) | 39.4 | 36–62 | 63.5 |
| Counts | |||
| Dorsal fin rays | II-27 | II, 27–30 | I, 27 |
| Anal fin rays | 25 | 24–26 | 25 |
| Pectoral fin rays | 11 | 10–12 | - |
| Caudal fin rays | 12 | 12 | - |
| Vertebrae | 20 | 20 | - |
| Measurements | |||
| Ratio of standard length | |||
| Body depth | 1.4 | 1.4–1.5 | 1.4–1.5 |
| Head length | 3.3 | 2.8–3.3 | 3.2–3.4 |
| Snout length | 4.2 | 4.0–4.8 | - |
| Soft dorsal fin base | 2.2 | 2.1–2.3 | - |
| Ratio of head length | |||
| Eye diameter | 2.6 | 2.6–3.4 | - |
| Gill slit length | 5.0 | 4.2–5.9 | - |
| First dorsal spine length | 1.3 | 1.2–1.5 | - |
| Interdorsal space | 1.2 | 1.2–1.5 | - |
| Longest soft dorsal ray | 3.4 (8th) | 2.9–4.3 (8th) | - |
| Caudal-fin length | 1.0 | 1.0–1.3 | - |
| Caudal peduncle depth | 2.0 | - | 1.8 |
| Caudal peduncle length | 2.6 | 2.5–3.7 | 2.3 |
| Ratio of Caudal peduncle depth | |||
| Caudal peduncle length | 1.3 | 1.3–1.4 | - |
Body color light brown or yellowish, and lower part of the belly light green. Body has black lines and black dots connected vertically. Three black lines both between the eye and mouth, and between eye and dorsal part. Several black dots and two black lines stretch from eye to bottom of pectoral fin base and gill slit. Black dots vertically connected from the mouth to caudal part through below pectoral fin, while black dots at upper part of pectoral fin connected from behind of eyes to caudal part. These vertical patterns mostly in a line at front body part and in dots at behind of body toward caudal part. Small hexagonal patterns appear on lower rear body as a dotted line. First dorsal spine light green, all soft rays generally transparent with light green or light blue.
Brachaluteres ulvarum is restrictly distributed in parts of the Northwest Pacific region, especially off Japan (Matsuura et al., 1984; Hayashi, 2002) and Korea (present study).
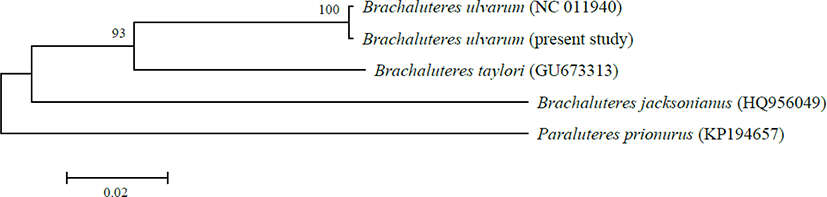
As a result of comparing the mitochondrial cytochrome c oxidase subunit I gene (COI) sequence (611 bp), present specimen with the mtDNA COI region of B. ulvarum registered in NCBI, the genetic distance of the two individuals was found to be 0.002. And another two species in genus Brachaluteres fishes with genetic distances of 0.088–0.164 (Fig. 3).
Discussion and Conclusion
The morphometrics of the specimen is well matched the original description of B. ulvarum given by Jordan & Fowler, 1902. However, compared with the holotype, there was a difference in the number of dorsal fin spines and in the caudal peduncle length. The second dorsal fin is very small and difficult to identify. In this study, it could be confirmed through x-ray. In addition, the difference in caudal peduncle length is thought to be a difference according to growth, and it is judged that the morphology measurement through more individuals is necessary in the future. The presence of two dorsal fin spines, and the range of the caudal peduncle length is well matched with the description suggested by Hutchins & Swainston (1985).
The genus Brachaluteres contains 4 species globally, and it forms small populations (Akagawa et al., 1995; Nelson et al., 2016). B. ulvarum occurs in southern Japanese waters from Wakayama Prefecture to Chiba Prefecture, including Sagami Bay and the Sagami-nada Sea, while B. jacksonianus (Quoy & Gaimard, 1824) in eastern Indian Ocean and Australian water, B. tayloriWoods (1966) in western Pacific, and B. fahaqaClark & Gohar (1953) in western Indian Ocean (Red Sea) (Froese & Pauly, 2021; Hutchins & Swainston, 1985; Matsuura et al., 2019).
The occurrence of B. ulvarum in the Korean waters is unexpected, because the species has only been reported from the Japanese waters (Hutchins & Swainston, 1985). In addition, there have been no previous records of the members of the genus Brachaluteres from Korean waters (MABIK, 2020). Therefore, our finding is the first documental record of Brachaluteres species in Korean waters. The data is also significant understanding additional zoogeographical pattern in the population of this species within Korean waters, filling the geographic gap its distribution in Northwest Pacific region.
There are several factors that might stop recording B. ulvarum from Korean waters previously. Among these, we can reside on (i) the lack of sampling in the area prevents the regular detection of this species in the Korean waters and has been overlooked in the past; (ii) due to global change, a recent natural colonization within Korean waters has been taken place; (iii) Korean waters is considered as one of the busiest waterways in the world and ballast water from ships is a possible; (iv) it is rare in the area.
Through this study, a record of B. ulvarum from the southern Korean water was given. In addition, its species verification as B. ulvarum was made by molecular study using mtDNA COI region (611 bp) as well as by its potential distribution range and body morphometrics. Finally, we propose the new Korean names “Kko-ma-jwi-chi-sog” for the genus Brachaluteres, and “Kko-ma-jwi-chi” for the species B. ulvarum because four species belonging to these genus have a maximum size of less than 10 cm TL, also, this species is the largest of which is 7.5 cm SL, and is small fish species.
