Introduction
Fleshy shrimp Penaeus chinensis is one of the most important commercial species in Korea and China and considered particularly valuable as they are found only in the Yellow Sea.
The fleshy shrimp production, however, sharply decreased in the mid-2000s due to the white spot disease outbreaks leaving capture production as the only option for the supply. The fleshy shrimp industry is now eager to restore the species as a viable aquaculture species. In Korea, there are major cultured shrimp species including non-native whiteleg shrimp Penaeus vannamei as well as native Kuruma shrimp Penaeus japonicus and fleshy shrimp P. chinensis. It is reported that the Kuruma shrimp has a long spawning cycle with multiple spawns while the fleshy shrimp exhibits a short spawning period of 1–2 months allowing only a single spawning event during its whole life (Cha et al., 2002; Garcia, 1977). The single short spawning in the fleshy shrimp poses challenges for an abundant seedling production.
Fundamental research on reproduction is important to improve the understanding of the reproductive processes of broodstock in captivity. The use of domesticated broodstock as opposed to captive wild broodstock may help the industry produce pathogen-free larvae, representing one of the most important strategies for successful shrimp farming activities. Several factors have a high impact on egg quality and quantity such as broodstock age and/or size, broodstock origin, type of endocrine manipulation, genetic variation and broodstock nutrition (Racotta et al., 2003). An adequately formulated maturation diet that meets the nutrient requirements of shrimp broodstock is the one of the most important criteria that determines a successful shrimp reproduction. Many investigations have been carried out to study various nutritional factors that play critical roles in the stimulation of shrimp sexual maturation, enhancement of fertility and the production of viable, high-quality offspring (Chimsung, 2014). Several studies have indicated that fresh feeds (e.g., clam, squid, polychaetes, Artemia) can promote a successful reproductive performance of shrimp broodstock. However, fresh feeds also present several disadvantages such as an increased risk of disease transmission, variable nutritional quality, unpredictable supply and the potential to cause deterioration of water quality in hatchery culture systems (Harrison, 1990; Harrison, 1997). The use of commercial diets is advantageous because they are easier to manage and store as well as present a lower risk of pathogenic contamination (Meunpol et al., 2005; Wouters et al., 2002). However, the lack of clearly defined nutritional requirements for black tiger shrimp Penaeus monodon broodstock presents a knowledge gap for adequate diet formulation and, thus far, results have been unsatisfactory when compared with fresh feeds (Meunpol et al., 2005; Wouters et al., 2002).
The present study aims to assess the potential of various feeds for maturation to induce multiple spawning events in the fleshy shrimp within their short spawning period by comparing the hematological response and the ovary re-maturation in shrimp fed with different experimental feeds including manufactured diets consisting of dried polychaetes, squid, and clam ingredients, a fresh animal diet, and a commercial maturation diet.
Materials and Methods
The experiment to evaluate the effects of diet on the ovary re-maturation of fleshy shrimp lasted 15 days. Five diets were used: fresh food which consisted of Pacific flying squid Todarodes pacificus, Manila clam Ruditapes philippinarum, air-dried marine polychaetes (Nereis virens, Delta Farms, Netherlands), commercial diet (Breed-S, INVE Aquaculture, Belgium) and three manufactured diets (unpublished data). Proximate composition (moisture, crude protein, crude lipid and crude ash) of the diets were determined through the standard methods of AOAC (1995). The analyzed content is presented in Table 1.
The fleshy shrimp broodstocks (mean body weight 89.2 ± 10.3 g, mean body length 24.6 ± 0.9 cm, Carapace length 5.4 ± 0.3 cm, gonad somatic index [GSI] 17.9 ± 1.38%) were collected from Narodo offshore and transferred to the laboratory. During a 4-day acclimation period, all the shrimp were fed the polychaetes diet. At the beginning of the experiment, 10 individuals were randomly distributed into 15 tanks (250 L aquarium). Each tank was randomly allocated to each dietary treatments in triplicates. Shrimp were fed three times a day (10:00, 18:00, and 24:00) using feeding trays. Leftover feed was removed before next feeding. Continuous aeration was provided to maintain a dissolved oxygen level of 7.0 mg/L, and the water temperature was maintained at 20 ± 1°C. The natural photoperiod was provided through the window. Water quality management was performed through 50% to 80% water exchange using reserve tanks.
The final body weight was measured to the nearest 0.1 g using a digital electronic balance. The GSI was recorded for morphological analysis according to the methods of Hidir et al. (2018) by weighing the ovary tissue and the total body weight of each shrimp.
The hemolymph of five shrimp selected from each tank was sampled using a 1 mL syringe coated with an anticoagulant solution. The serum was centrifuged at 1,000×g 4°C for 10 min and then the plasma supernatant was collected without disturbing the hamocyte and frozen at –80°C until analysis by enzyme-linked immunosorbent assay.
Total lipid was analyzed using a lipid assay kit (Abcam, England) according to the manufacturer’s manual. Total protein, glucose and cholesterol were analyzed by an enzyme activity-based clinical kit (Asan Pharm). Hemocyanin (mmol/L) concentrations were determined from10 μL of hemolymph diluted in 990 μL of distilled water in a quartz cuvette. Absorbance at 350 nm was measured with a spectrophotometer (Bio-Rad), manually calibrated with distilled water (Gómez Bonilla et al., 2012). The final concentration was determined according to Chen & Cheng (1993) using the coefficient of extinction of hemocyanin (17.26) and factor of dilution.
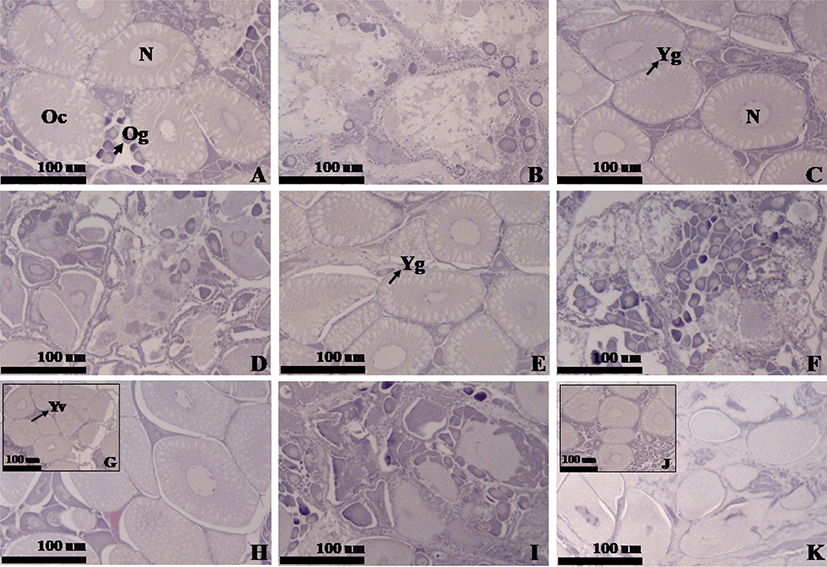
The histological study was done based on the standard histological procedure from Mumford (2004). Ten shrimp sampled from each treatment were dissected and the parts of the ovaries tissues were fixed in Davidson’s fixative solution. Routine thin section paraffin histology was performed at 5 μm thickness on a microtome and attached to a slide using Mayer’s albumin. Each of the sections was then dewaxed, dehydrated, and stained with hematoxylin-eosin. The slides containing these sections were then mounted with dibutylphthalate polystyrene xylene to be observed under an advanced research microscope (Carl Zeiss Z1, Oberkochen, Germany) together with AxioVision Imaging Software. The histological assessment of ovary indicated the presence of four different stages the previtellogenesis, growing stage, maturation and spawning stages.
Results from the feeding trial were analyzed by one-way analysis of variance (ANOVA) using a statistical software package called Statistic (version 3.1, Analytical Software, Heidelberg, Germany) to test effects of the dietary treatments. When a significant treatment effect was observed, the least significant difference test was used to compare means. Significant difference among the treatments was considered at α = 0.05.
Results and Discussion
Proximate analyses of the manufactured and commercial diets ingredients showed that moisture, crude protein, crude lipid, and crude ash contents were significantly different between the diet treatments. A comparison with the proximate composition of fresh feed ingredients–polychaetes (P), squid (S), and clam (C) (Table 1) revealed that the fresh ingredients had a higher protein and a lower lipid level compared to the commercial diet (Table 2).
The 15-day feeding trial in which the fleshy shrimp were fed with different maturation diets revealed that the experimental animals had a similarly high preference for the manufactured and fresh diets (both 100% consumed), but a lower preference for the commercial diet (50%–75% consumed). As semi-moist diet, the commercial feed crumbles more easily in water than dry diets, and the commercial feed treatment required daily 8 times of water exchange during the experiment. The other treatments received only twice a day of water exchange with no water quality issue.
At the end of the experiment, the GSI was the highest in the fresh diet group at 4.3%; the spawning volume in the manufactured diet of P + S at 134,000; and the hatching rate-the percentage of fertilized eggs that develop into nauplius was significantly lower in the manufactured diet of P + C (Table 3). Cahu et al. (1994) and Cavalli et al. (2000) reported a higher frequency of spawns with higher dietary levels of phospholipid.
In this study, hemocyanin, glucose, cholesterol were not significantly different between the treatments. But the total lipid was significantly higher in the P + C and P + S + C groups than the other experimental groups (Table 4). Several authors have reported a decrease in total lipids in the hepatopancreas and hemolymph during maturation, and assume they are transferred to the ovary (Castille & Lawrence, 1989; Millamena & Pascual, 1990). Palacios et al. (2000) reported that the ovary may need to reach a certain level of reserves to mature and spawn. If these levels are not reached, instead of spawning, the ovary may enter a reabsorption phase. The high total lipid levels in P + C and P + C + S groups were expected that multiple spawns (Table 4).
The histology analysis revealed three different stages of growing stage, maturation and spawning stages, each of which is characterized in Fig. 1. The growing stage in Fig. 1G generates yolk granules within vesicles of the cytoplasm with the yolk starting to accumulate. The maturation stage in Figs. 1A, 1C, 1E, 1H, and 1J, shows developing oocytes and a number of yolk granules concentrated in the cytoplasm. The stage initially exhibits a row of cortical alveoli along with the oocyte membrane, which are gradually expanded into two to three rows and occupy the most cytoplasm (matured egg diameter: 100–150 μm). In the spawning stage shown in Figs. 1B, 1D, 1F, 1I, and 1K, egg capsules consisting thin layers of connective tissue are identified and numerous oogonia are generated from germinal epithelium (ooctye diameter: 15–20 μm). According to the ovary histology analysis, the ovaries of the P + S treatment were in the most advanced stage of spawning and those of the P + C and P + S + C groups were in the maturation stage (Table 5 and Fig. 1). The ovaries of the commercial feed group were in the growing stage, indicating a slower re-maturation compared to the other treatments. This result is assumed to be associated with the lower utilization (preference) of the commercial diet by shrimp. The lower preference for the commercial feed led to a poorer nutrition in the shrimp affecting on the ovary maturation length.
| Stages | Manufactured feed | Commercial feed | Mix of animal feed | ||
|---|---|---|---|---|---|
| P + S | P + C | P + S + C | |||
| Previtellogenesis | - | - | - | - | - |
| Growing stage | 20 | ||||
| Maturation stage | 40 | 60 | 60 | 40 | 40 |
| Spawning stage | 60 | 40 | 40 | 40 | 60 |
Continuous forced reproduction has been suggested to produce exhaustion of the female reserves because of insufficient time between spawning to allow the shrimps to accumulate sufficient nutrients (Aquacop, 1977; Beard & Wickins, 1980; Browdy, 1992; Harrison, 1990; Primavera, 1984). If this is true, females with multiple spawning would be expected to have diminished reserves, especially in the ovary, and as a consequence, to have an inadequate maturation process. If a female with diminished reserves is forced to mature, it may produce eggs of lower quality. It has been reported the first spawning of a female is the best in terms of fecundity, fertilization, hatching, and metamorphosis rates (Beard & Wickins, 1980; Braga et al., 2010; Bray et al., 1990; Emmerson, 1980; Marsden et al., 1997; Palacios et al., 1998).
Conclusion
The fleshy shrimp live one year from the hatching in June to the spawning in next May to June (Lee et al., 2012). The spawning comes about one month earlier than that of Shiba shrimp Metapenaeus joyneri, Southern rough shrimp Trachypenaeus curvirostris, and Kishi velvet shrimp Metapenaeopsis dalei and lasts only about one month (Cha et al., 1997; Choi, 2001).
The combined results of the hematological and histological analyses of the experimental shrimp showed that the manufactured, fresh, and commercial diets were all effective for shrimp re-maturation indicating a great potential of multiple spawning depending on the availability of maturation feeding during the main spawning period. It is expected that the manufactured maturation diets could replace fresh feed for a more effective management of water quality and disease control. It is also expected that further research on early maturation and multiple spawning using the manufactured diets would contribute to developing a shrimp culture technique for early seedling production and shrimp domestication.
