Introduction
Pacific white shrimp (Penaeus vannamei) is one of widely popular shrimps all over the world because of its unique flavor and nutritional value. It has been well known that the appearance of shrimps is important to show its freshness. When shrimps are stored improperly, discoloration of shrimp would happen due to deterioration. This process is called melanosis (Arancibia et al., 2015). Melanosis is a biochemical process induced by oxidation of polyphenol oxidase (PPO). PPO can oxidize tyrosine into quinones in shrimps. PPO combines with protein to produce melanin, resulting in black spots on shrimp surface and affecting its sensory quality. Pacific white shrimp is one of the most popular shrimps in China, but the quality and revenue of shrimp would be greatly reduced by melanosis during transportation and sales.
At present, there are melanosis inhibitors such as sulfite (Galvão et al., 2017) and 4-hexylresorcinol (Surasani et al., 2012). However, these melanosis inhibitors not only threaten the health of consumers, but also have a negative effect on the sensory characteristics of aquatic products. Currently, SO2 produced by decomposition of sodium metabisulphite (SMS) is widely used as a common shrimp melanosis inhibitor. SO2 generated by SMS will remain in food, causing allergic reactions, as well as potential health hazards to consumers.
Hypotaurine (HTU) is a derivative of non-protein amino acid that is widely found in crustaceans. It can be transformed to taurine in human body. Taurine is known as conditionally essential amino acids, which is non-toxic and harmless. HTU has antioxidant properties (Aruoma et al., 1988). It can neutralize hydroxyl radicals produced by oxidation and avoid oxidative damage (Ortega et al., 2008). HTU and SMS can oxidize the tetravalent sulfur to hexavalent sulfur. HTU does not produce SO2 and has no smell does not affect food flavor. Studies have shown that HTU inhibits enzyme activity by affecting the structure of PPO (Zhou et al., 2021). It has been used as the preservative in grape juice, peach and yam (Schulbach et al., 2013). Because of the HTU’s dual effects of anti-oxidation and anti-enzymatic browning, it has a potential property in delaying the melanosis and improving quality of aquatic products.
There would be a great loss in shrimp production if melanosis occurs. Therefore, a safe, natural, and efficient melanosis inhibitor is sought to prolong the shelf life of shrimps. Although, HTU has been used in the preservation of fruits and vegetables, but there are few studies on the preservation effect of shrimps particularly on melanosis and quality changes of shrimp during storage (Liu et al., 2022). Hence, this research is to determine the effect of HTU treatments at different concentrations on melanosis and physicochemical properties of the white shrimp P. vannamei. This study aims to provide valuable information on the use of HTU in the preservation of shrimp products.
Materials and Methods
2,2-Diphenyl-1-picrylhydrazy (DPPH) free-radical scavenging assay method is used to evaluate the antioxidant activity of HTU. The DPPH radical scavenging capacity was measured according to the method of Singh et al. (2002). HTU was dissolved in deionized water to prepare sample solution (0.05%, 0.1%, 0.5%, 1%, 1.5%, and 2% [w/v]). Then 2 mL of sample solution and 2 mL of DPPH (concentration: 0.2 mmol, dissolved in absolute ethanol) were mixed homogenously. The mixture was incubated in the dark at 37°C for 30 min. The absorbance of the solution was measured at 517 nm. Alcohol was used as the blank control and vitamin C was used as the positive control. DPPH radical scavenging rate was calculated according to the following formula:
Where: Ai, sample absorbance; Aj, background absorbance; A0, control absorbance.
The following experiments were carried out using HTU with representative antioxidant results. Shrimps were purchased from the local shrimp farmer in Hangzhou, China. Shrimps were washed immediately with ice water and soaked at 4°C for 30 min with HTU solutions (1, 10, and 20 g/L). Shrimps treated with distilled water was used as the 0 concentration control, and SMS solution (12.5 g/L) was used as the positive control (Nirmal & Benjakul, 2009). The ratio of shrimp/solution was 1:2. Then the shrimps were taken out and drained, packed in a sterile polyethylene bag and stored at 4°C. They were randomly selected from each treatment on day 0, 2, 4, 6, 8, and 10 for physical and chemical, microbial, and sensory analysis.
The internal changes of shrimp can be expressed by melanosis score and chromatic aberration. Referring to the method of Montero et al. (2001) of 10 point scoring standard, five professional expert members were invited to evaluate melanosis of shrimps through visual inspection and gave score of 0−10, of which 0 indicated no melanosis of shrimps, 10 points indicated that the shrimp body completely turned red and black.
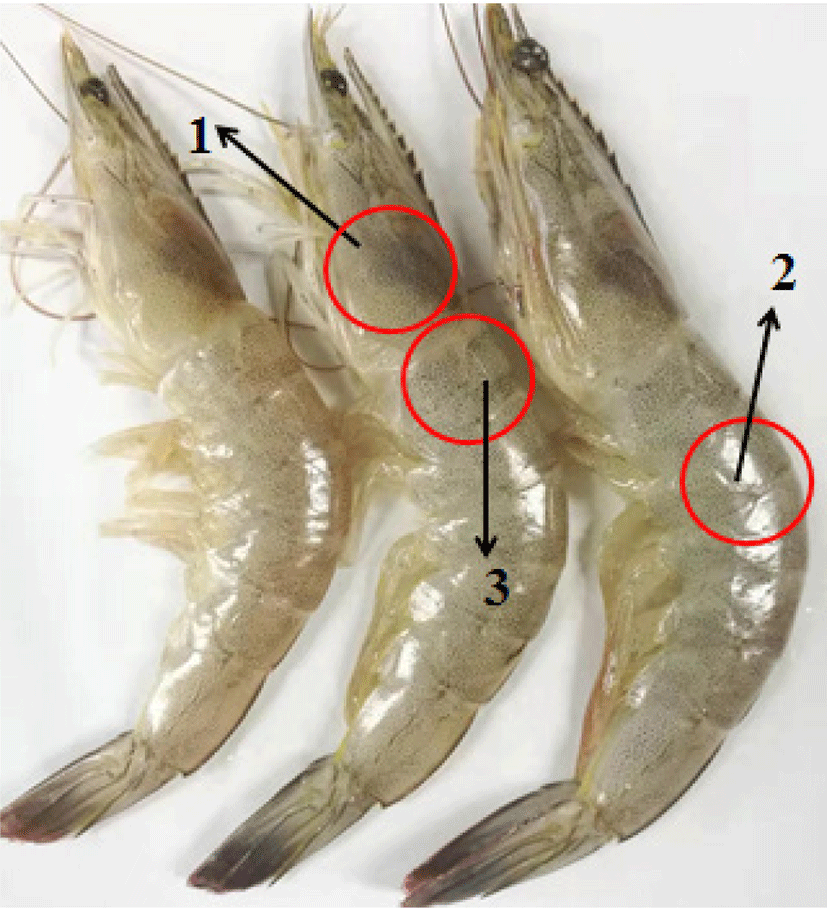
The color change of shrimp was measured by a hand-held color meter (CR-410, Konica Minolta, Shanghai, China). Based on the measured value of CIE LAB, the color was determined in the cephalothorax of shrimp (Fig. 1, mark 1). ∆E calculated according to the following formula:
The pH of shrimp meat is one of the important indicators to detect the freshness, and the change of total volatile basic nitrogen (TVB-N) content causes the rise of pH. Referring to the method of pH by Aro et al. (2010), the 1 g of shrimp meat was homogenized at 15,000 rpm for 2 min with 30 mL of deionized water. Then the mixture stabilized for 30 min. The homogenate was used to pH value measurement with the pH meter (EF-20K, Mettler Toleclo, Zurich, Switzerland).
Referring to the method of TVB-N by Sallam (2007), the 2 g of shrimp meat mixed with 30 mL 20 g/L TCA in a 50 mL centrifuge tube. The mixture was homogenized at 15,000 rpm for 2 min, and then centrifuged at 4,800×g for 10 min. Take the supernatant to 50 mL with TCA, 5 mL of mixture was put into the digestive tube and was measured the TVB-N value with Automatic Kjeldahl nitrogen meter (ZDDN-II, Deka precision meter, Shenzhen, China).
Total bile acid (TBA) value was used to reflect the level of malondialdehyde (MDA) which is a secondary metabolite formed by fat oxidation, and is an important index for evaluating lipid oxidation of aquatic products. According to the method of Wu et al. (2016), the 3 g of shrimp meat mixed with 30 mL of 7.5% (w/v) TCA (containing 0.1% EDTA), was homogenized at 15,000 rpm for 2 min and then centrifuged at 1,600×g for 10 min. After taking 5 mL of supernatant and adding 5 mL of 0.02 mol/L TBA solution. The mixture was cooled down in ice water to room temperature after 40 min of water bath at 90°C. The absorbance value of the sample was measured at 532 nm with distilled water as blank. The standard curve was prepared with 1,1,3,3-tetraethoxypropane standard. The result was expressed as the milligram of MDA contained in 1 g shrimp meat (mg MDA/kg).
The small pieces (5 × 5 × 2 mm) cut from the first abdominal muscle of shrimp (Fig. 1, Mark 3) was soaked in 3% glutaraldehyde at 4°C for 24 hours. The samples were dehydrated with ethanol and embedded in paraffin. Then the paraffin block was cut for cross-section and longitudinal section. After staining with hematoxylin and eosin, the microstructure of the sample was photographed and observed by optical microscope.
Texture can reflect the quality of shrimps. Referring to the method of Valenzuela-Melendres et al. (2014), the second abdominal muscle of shrimps was taken to measure the hardness and springiness (Fig. 1, Mark 2), with the P/5 probe. And the speed was 1 mm/s, the shape variable was set to 50% (TAXI2i, Stable Micro Wystem, Surrey, UK).
Shrimp is rich in nutrition, which are easy for various microorganisms to breed and reproduce during storage. Therefore, the freshness of shrimps can be judged by the determination of total plate count (TPC). TPC was determined according to the method of Wu et al. (2017) and partially modified. The whole process was carried out in a sterile environment. The 10 g of shrimps’ meat was mixed with 90 mL of 0.85% sterile normal saline. A 100 μL appropriate dilution gradient of bacterial solution was added to the plate by coating. TPC was calculated after cultured at 37°C for 48 hours.
Referring to the method of Li et al. (2013), the 10 professionally trained sensory assessors were selected to score from three aspects: appearance, smell, and texture. The scoring range of each parameter was 1−10, of which 10 was the highest quality sample.
One-way analysis of variance (ANOVA) was performed using a SPSS 24.0 package (IBM, Armonk, NY, USA). A significant difference was determined at the 0.05 probability level, and differences among the mean values of various treatments were measured by Duncan’s multiple range test (p < 0.05). ANOVA was carried out in antioxidant parameters and color. All measurement steps were repeated for 3 times, and parallel experiments were performed in triplicates. The results were expressed in the form of mean ± SD. The figures were drawn by origin 8.3 (OringinLab, Northampton, Massachusetts, USA) software.
Results and Discussion
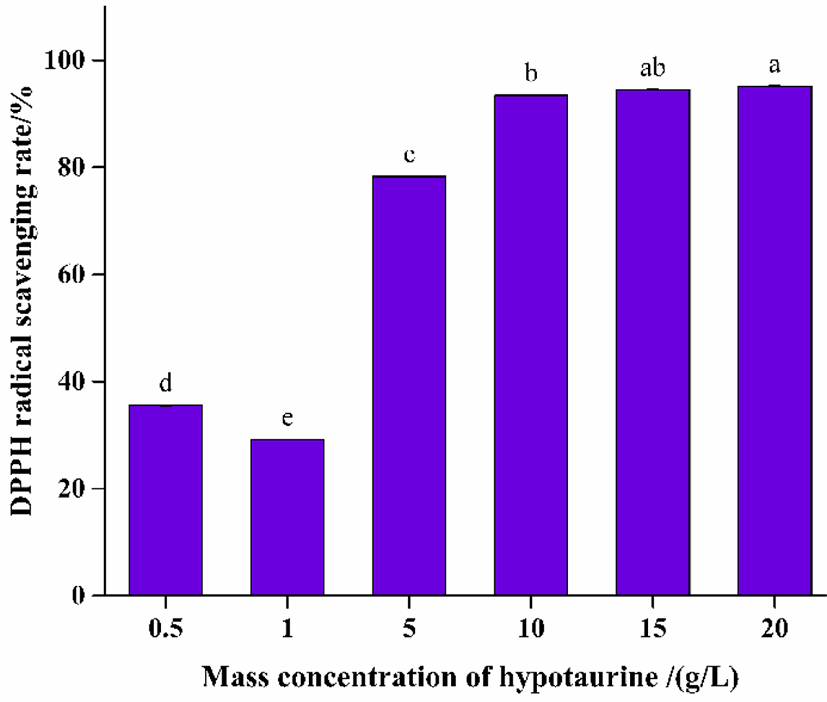
As shown in Fig. 2, DPPH radical scavenging rate increased with the increase of HTU concentration (p < 0.05). This result was consistent with the report by Zhang et al. (2019). Studies had shown that sulfinates could be oxidized to sulfonyl by hydroxyl radicals and further oxidized to sulfonates with oxygen consumption, thus sulfinates could effectively remove hydroxyl radicals in cells and act as antioxidants to protect cells from oxidative damage. In this study, there was no significant difference in the free radical scavenging rate of 10 and 20 g/L HTU, which was related to the ultimate free radical scavenging capacity of HTU. The free radical scavenging rate of 12.5 g/L SMS treatment group was 89.37%, which was lower than 93.45% of 10 g/L HTU treatment group. These results showed that HTU on high concentration showed better results of free radical scavenging and have potential effect on preventing melanosis of shrimp.
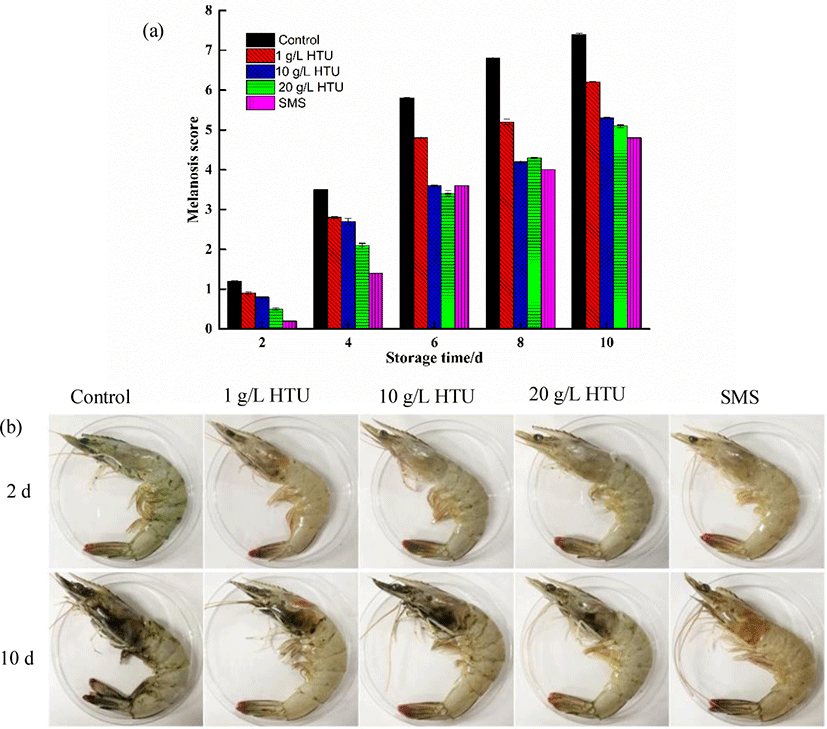
Melanosis changes of shrimp during refrigeration at 4°C was shown in Fig. 3a. The increase rate of melanosis varied with different treatments (p < 0.05). At day 2, melanosis was not obvious in all samples. After day-8 of storage, melanosis progression in control and 1 g/L HTU treatment were notable (control = 6.8, 1 g/L HTU = 5.2), and significantly higher than the other treatment (20 g/L HTU = 4.3, 10 g/L HTU = 4.2, 12.5 g/L SMS = 4.0). At day 10, a severe melanosis was observed in the control and 1 g/L HTU treatment, while the melanosis intensity of shrimp treated with 10 g/L HTU, 20 g/L HTU, and 12.5 g/L SMS was moderate. Fig. 3b displayed a photograph showing progression of melanosis. After 10 days of storage, shrimps in control treatment and 1 g/L HTU treatment not only showed melanosis, but also showed weak connection between shrimp head and shrimp body, and internal substance of shrimp head overflowed. These phenomena indicated that the quality of shrimp had been seriously degraded and was considered to be completely inedible. However, shrimps treated with 10 g/L and 20 g/L HTU showed only mild melanosis in the head and tail of the sample shrimp. In previous experiments, we obtained that the inhibition type of HTU on PPO activity is competitive reversible inhibition, which caused the change of enzyme conformation by binding with the active site of the enzyme, resulting in the decrease of enzyme catalytic activity. High concentration of HTU bound more to enzyme sites, so it could inhibit melanosis better (Zhou et al., 2021). Shrimps treated with 12.5 g/L SMS showed slight redness and melanosis, which might be related to the release of astaxanthin (Ferrer et al., 1989).
Chromatic aberration is also used to assist in the evaluation of melanosis. The changes of lightness (L*), yellowness (b*), redness (a*), and total color difference (∆E) in shrimps during storage were shown in Table 1. With the extension of storage time, the L*-value of shrimps decreased, and b*-values increased, meaning that melanosis occurred obviously in shrimp, resulting in the decline of brightness. The L*-values and b*-values of HTU treatments were generally better than that of the control treatment, which was supposed due to its ability to inhibit the activity of PPO, preventing the polymerization of carbonyl compounds (Martı´nez-Alvarez et al., 2007). The change of L*-value in SMS treatment was the slightest. It might be the strong reducibility of SMS, which reduce the o-quinones generated by enzymatic Browning to phenols. The a*-values showed a gradually rising trend over time during storage. During storage, with the propagation of microorganisms and the action of enzymes, protein denaturation occurred, causing astaxanthin to dissociate from the protein-astaxanthin complex and presenting the original red color. HTU had better antioxidant capacity, which could slow down the oxidative denaturation of protein. The a*-value of HTU treatments were significantly lower than that of the control treatment and SMS treatment (p < 0.05). The SMS treatment showed the highest value of redness. It was speculated that SMS could react with amino acids, proteins and other substances to generate disulfide bond compounds, so it was easy to denatured shrimps protein, resulting in the redness of shrimps (Qian et al., 2013). ∆E-value could reflect the total color changes of shrimps. Compared with the control treatment, the ∆E of HTU increased more slowly. This result was similar to research of López-Caballero et al. (2007). This result was related to the fact that HTU could inhibit the discoloration of shrimp during storage by inhibiting PPO and antioxidant capacity.
The change of pH could be seen from Fig. 4a. The pH value decreased first and then increased with the extension of storage time. The decrease of pH might cause by glycolysis of cells under anaerobic respiration after the death of shrimps. With the action of endogenous enzymes and microorganisms, the protein degraded and produced alkaline substances such as ammonia compounds and trimethylamine, leading to an increase in pH value (Liu et al., 2018). At day 7, pH of the control treatment (7.78) exceeded the consumer acceptable limit value of 7.6, indicating that shrimp had completely undergone spoilage (Wu, 2014). The pH value of the two HTU treatments with high concentration (10 and 20 g/L) increased to about 7.5−7.6 at the last day. This indicated that after soaking with the solution of HTU, the spoilage and deterioration of shrimps during storage could be delayed obviously. This might be that HTU had potential antibacterial effect, and this effect increased with the increase of concentration. The pH of SMS treatment reached the limit value on the 10th day of storage. The reason might be that SMS reacted with proteins, which accelerated the denaturation and degradation of proteins, and led to the increase of alkaline substances (Ojagh et al., 2014).
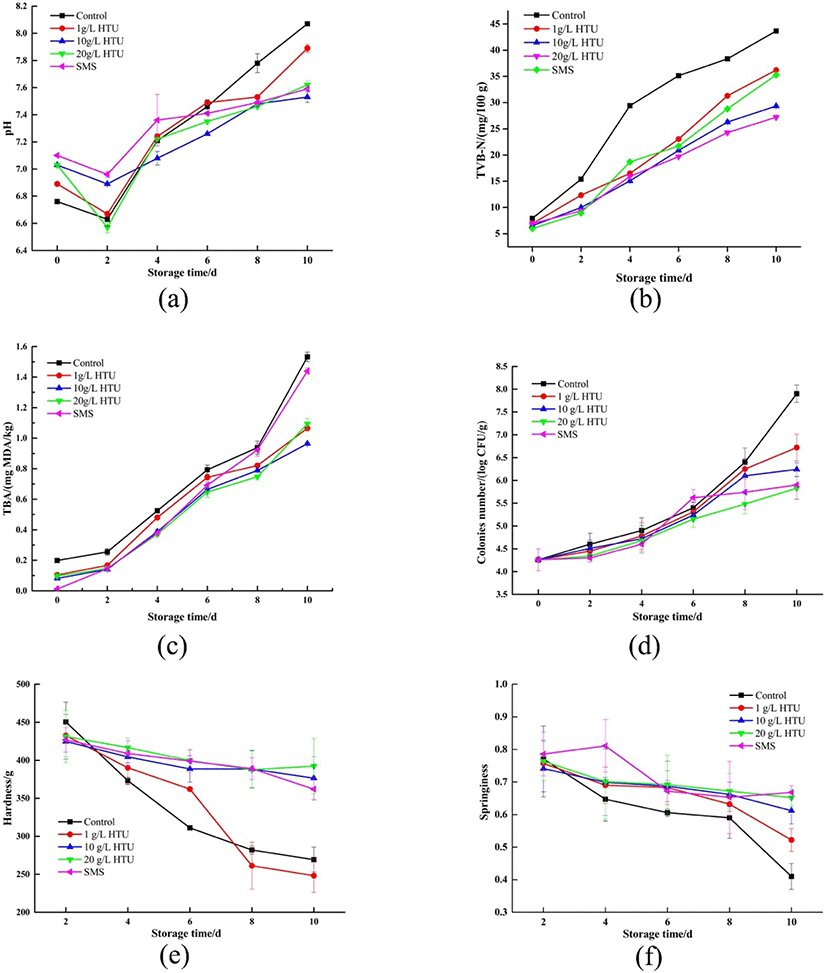
The to TVB-N refers to the content of volatile basic low molecular amines produced by proteins due to the action of microorganisms and enzymes (López-Caballero et al., 2007). In general, only when the TVB-N value of seawater fish and shrimp is ≤ 30 mg/100 g can it be regarded as qualified products (Guan et al., 2019). As shown in Fig. 4b, TVB-N values showed an increasing trend with the extension of storage time, but the increase rates of different treatments were different. The TVB-N content of the initial fresh shrimps was 7.91 mg/100 g. At day 6, the TVB-N value of the control treatment had reached 35.13 mg/100 g, which was completely inedible. The increase of TVB-N in 20 g/L HTU treatment was the least. It can be attributed to the inhibitory activity of HTU against TVB-N-producing bacteria and proteolytic enzymes. In general, immersion preservation of shrimp by 20 g/L HTU treatment could indeed reduce the production of TVB-N.
Shrimp contains lots of unsaturated fatty acids, which are prone to fat oxidation and hydrolysis to produce ketones and aldehydes during transportation and processing, resulting in an unpleasant smell of corruption (Chaijan et al., 2006). The higher the TBA value is, the higher the degree of fat oxidation. Once the TBA exceeds 1.00 mg/kg, the meat is spoiled and is not edible (Bahmani et al., 2011).
The TBA of shrimp were shown in Fig. 4c. The TBA value of all treatments increased continuously during the storage period (p < 0.05). Fat oxidation occurred in shrimps during storage, which was positively correlated with time. On the 10th day, the TBA value of control treatment and SMS treatment was close to the limit of deterioration and inedibility, which was much higher than that of HTU treatment (p < 0.05). The degree of lipid oxidation in SMS treatment was like that in control treatment, suggesting that SMS would react with lipid to form sulfate. The group treated with different concentrations of HTU could extend to the 10th day, and there was no significant of TBA between different concentrations (p > 0.05). HTU had good antioxidant activity and could slow down the degree and rate of fat oxidation in shrimp (Bahmani et al., 2011), which was consistent with the results of DPPH free-radical scavenging assay.
Fig. 4d showed the change of TPC with an upward trend during storage. At the beginning, the slow growth of TPC might be due to the decrease of pH caused by glycolysis, which inhibited the growth of bacteria. TPC increased rapidly after glycolysis. At day 8, TPC in the control treatment (6.4 log CFU/g) had exceeded the edible safety limit of aquatic products by 6.00 log CFU/g (Mendes et al., 2014). TPC in 1, 10, and 20 g/L HTU treatments were 5.68, 5.48, and 5.37 log CFU/g at the last day. HTU could significantly delay the corruption of shrimp meat, two reasons were speculated. The first reason was that HTU could neutralize oxidizing substances released by microorganisms, such as free radicals, and slow down the impact of oxidative denaturation on protein and fat (Nirmal & Benjakul, 2011). The second reason was the inhibition of microbial enzymes, which reduced the activity of microorganisms. For example, the inhibition of PPO caused the change of enzyme conformation, resulting in the decrease of enzyme catalytic activity.
TPA analysis was carried out on shrimp in different treatments during storage. Hardness and springiness were selected to evaluate the quality changes of the shrimp. Hardness refers to the strength of deformation of food under certain external extrusion. The hardness decreased with the extension of storage time in Fig. 4e. This was possibly caused by two reasons: first, ice crystals were formed during freezing, which destroyed the muscle structures of shrimp; second, because of the role of microbial reproduction and enzymes, muscle tissue proteins were degraded, and the connection of tissue structure was fragile, resulting in the decrease of hardness. The hardness of control treatment and 1 g/L HTU treatment decreased the most rapidly, while the hardness of SMS treatment, 10 g/L HTU treatment and 20 g/L HTU treatment group obviously decreased slowly. They could reduce the oxidative damage of protein and maintain good tissue morphology because of good antioxidant ability (Pardio et al., 2011). Springiness defines the distance is measured when the food recovers its height within the time elapsed between the end of the first extrusion and the beginning of the second extrusion in TPA. It can be seen from Fig. 4f that the springiness decreased gradually with the extension of storage time. Compared with the control treatment, the springiness of HTU treatments decreased slowly, and there was no significant difference between different concentrations of HTU (p > 0.05). Shrimps treated with HTU had better springiness of muscle, which was consistent with the hardness trend. The parameters of muscle texture might be affected by many factors, such as the rate and degree of pH decline after death, the degradation of myofibrils and connective tissue proteins in shrimps (Sae-Leaw et al., 2017).
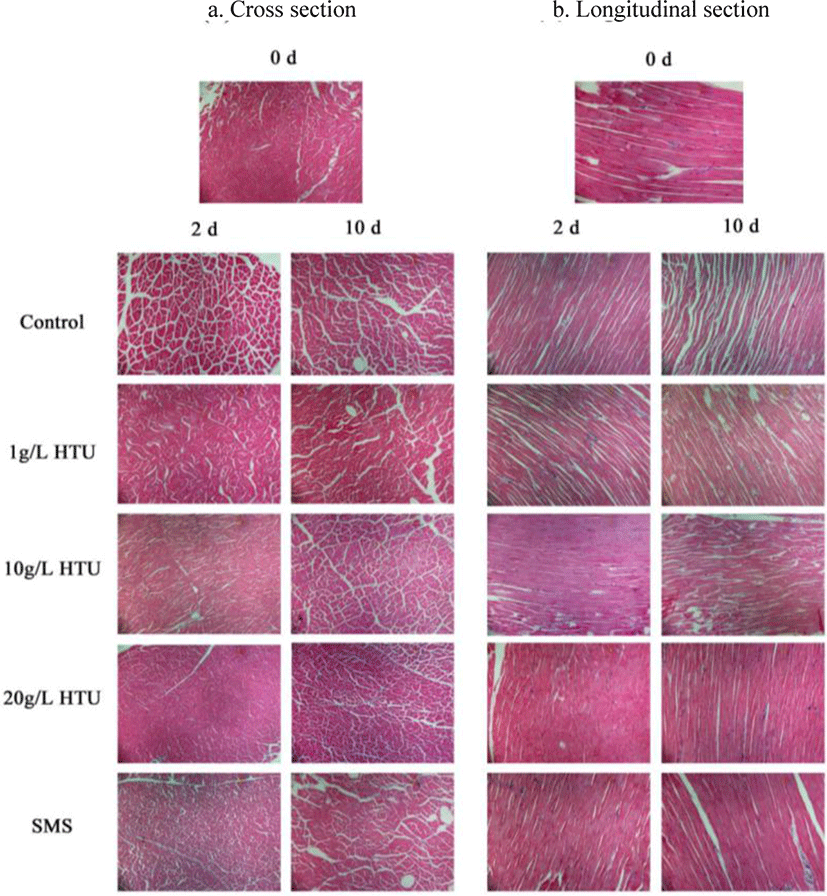
Fig. 5 showed the transverse and longitudinal sections of tissue sections of each treatment at day 2 and day 10. It could be seen that different treatments had a significant impact on the muscle tissue structure of shrimp. The muscle fibers of fresh shrimp were arranged compactly and orderly, the cells were complete, and no obvious gap was observed between cells. The gap between fresh shrimp cells in the control treatment was increased at day 2. At day 10, the control treatment had huge cell gap, separation of muscle fibers from sarcomeres, muscle filament tear, myofibril distortion, deformation and even fracture in the longitudinal section. The reason was that, without HTU, the degradation of myofibrils and internal connective tissue during freezing storage would lead to the separation of myofibrils from sarcomeres, the gap between myofibrils would increase, the degradation and denaturation of protein would also lead to the fracture of myofibrils (Allan Bremner & Hallett, 1985). At day 10, the microstructure of 20 g/L HTU treatment was dense, fresh and the quality was the best. This phenomenon showed that 20 g/L HTU could maintain the integrity of shrimp tissue. The result was consistent with the change of texture.
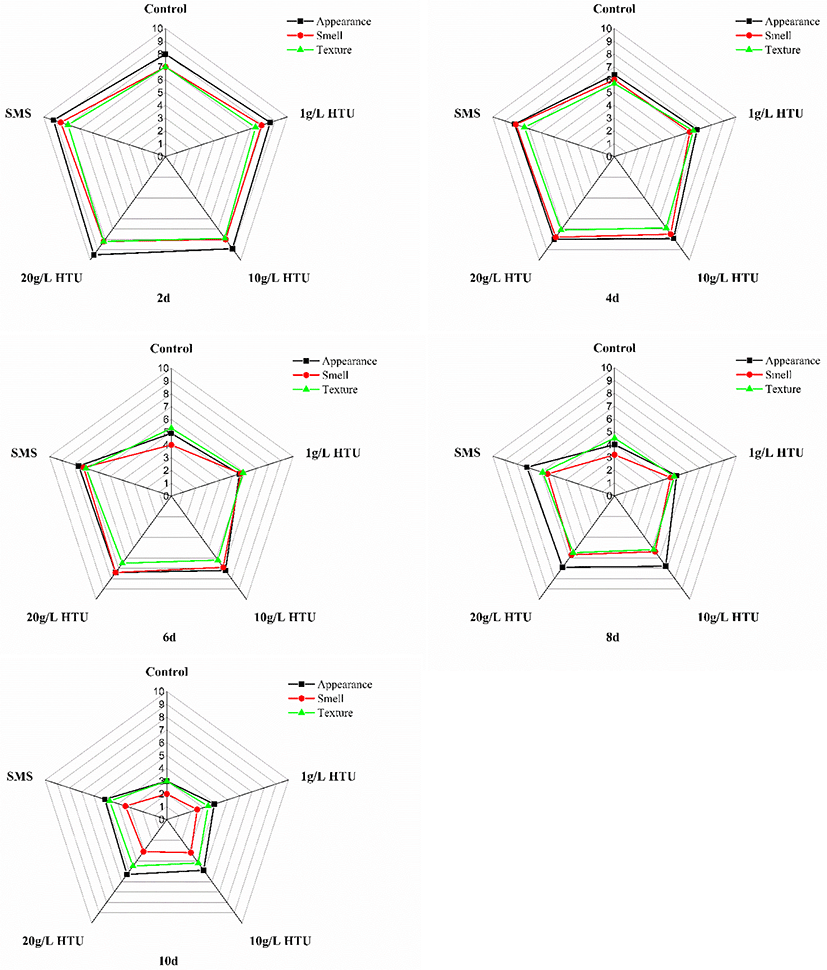
Sensory evaluation is the most intuitive evaluation index of aquatic products, which can comprehensively represent the changes of shrimp appearance, smell, and texture. Fig. 6 was the radar chart of sensory evaluation of shrimp on different treatments. The scores of appearances, smell and texture showed a downward trend. The sensory scores of the control treatment decreased rapidly. At day 6, the scores of the control treatment reduced unacceptable levels, resulting in an unpleasant smell and soft texture (appearance 4.9, smell 4, and texture 5.3). On the contrary, the 20 g/L HTU treatment kept well in the range of high score of sensory quality at day 8 due to its effective antioxidant and the inhibitory activity fraction of PPO. This result was consistent with the changes of appearance and texture parameters.
Conclusion
HTU had good antioxidant activity and could significantly inhibit the melanosis of shrimp. The inhibition efficiency of HTU depended on the concentration. The lipid oxidation, TPC and muscle structure loss of shrimp treated with 20 g/L HTU during storage were low (p < 0.05). Therefore, 20 g/L HTU may be a good choice, which shows great potential in controlling melanosis during storage and prolonging the shelf life of shrimps.
