Introduction
Probiotics are microorganisms that provide health benefits to their host without side effects. Particularly, Bacillus species have been successfully applied in aquaculture as probiotic agents (Kuebutornye et al., 2019). Dietary supplementation with Bacillus species can increase the production of anti-oxidant enzymes in host animals (Nayak, 2021). These probiotic microorganisms can minimize stresses in aquatic animals by modulating internal and external factors, i.e. chemical contaminants and pH in rearing tanks or ponds (Hlordzi et al., 2020). Bacillus species were reported to prevent tissue damage by reducing reactive oxygen species and transaminase enzymes (Kuebutornye et al., 2019). Bacillus species could also improve disease resistance of host animals by producing bacteriocins (Kim et al., 2014). Further, Bacillus species have been reported to enhance growth and feed utilization efficiency of aquatic animals (Nayak, 2021).
Bacillussp. SW1-1 (GenBank accession number: KF233990) is a probiotic isolated from shrimp intestines. Kim et al. (2014) reported that Bacillus SW1-1 exhibited antimicrobial activity against Edwardsiella tarda, Streptococcus parauberis, S. iniae, Vibrio anguillarum, and V. harveyi. They reported that the bacteriocin was responsible for the antimicrobial activity of the Bacillus SW1-1 without any harmful effect on some beneficial bacteria.
Olive flounder (Paralichthys olivaceus) is the most highly produced finfish species in South Korea. However, bacterial disease is one of the main causes for the production losses in olive flounder farms. Other diseases due to infections by E. tarda, S. iniae, and Flexibacter ovolyticus have been reported to damage the production output (Jung et al., 2020). It is well known that probiotics are reported to improve innate immune responses and disease resistance of olive flounder. Lactococcus lactis WFLU12, a common component of fish microbiomes, improved disease resistance against S. parauberis infection (Nguyen et al., 2017). Similarly, a mixture of probiotics enhanced the innate immunity of olive flounder against Uronema marinum infection. L. lactis BFE920 and Lactobacillus plantarum FGL0001 enhanced immunity and survival against S. iniae infection in olive flounder (Beck et al., 2015). Enterococcus faecium increased innate immune responses and protected the fish from Lactococcosis (Kim et al., 2012). Bacillussp. SJ-10 and L. plantarum upregulated the expression of immune genes and improved disease resistance against S. iniae challenge (Hasan et al., 2019; Jang et al., 2019).
Probiotics are often applied in aquaculture as a water treatment or administrated as feed additives (Chauhan and Singh, 2019). External coating is very useful method because it can provide benefits as both water treatment and feed additive. Therefore, this study sought to investigate the effects of dietary coating of Bacillussp. SW1-1 on growth performance, feed utilization, survival, and disease resistance of olive flounder. Additionally, the changes in hematological and innate immune parameters were monitored before and after E. tarda challenge.
Materials and Methods
The bacteria strain was cultured in an industrial culture broth which prepared in Woo Gene B&G’s Central Research Center (Hwaseong, Korea). The broth was spray-dried to obtain dry powder. The powder was mixed with glucose, starch and calcium carbonate mixture to contain 0.4% Bacillus powder in the mixture. The numbers of viable cells were 1.0 × 107 CFU/g in the mixture after culturing on tryptic soy agar (236950, BD Difco, NJ, USA) plates.
A commercial diet was used as the basal control (AP0) and two other diets were prepared by coating 0.25% (AP25) or 0.50% (AP50) Bacillus sp. SW1-1 mixture (SW1-1) onto the basal diet. For the coating, SW1-1 powder was dissolved in 100 mL distilled water to be 0.25% or 0.50% solution. The two diets (AP25 and AP50) were coated with the SW1-1 solution for 20 min while the basal diet was only coated with distilled water. Then, the coated diets were dried in an electric drier for 4 h and stored at −20°C until use. The proximate composition of the diets was provided in Table 1.
| Moisture | Crude protein | Crude lipid | Crude ash | |
|---|---|---|---|---|
| AP0 | 6.82 ± 0.10 | 51.8 ± 0.34 | 9.79 ± 0.43 | 13.0 ± 0.0 |
| AP25 | 7.73 ± 0.05 | 52.1 ± 0.06 | 9.64 ± 0.24 | 13.1 ± 0.0 |
| AP50 | 7.70 ± 0.02 | 51.9 ± 0.38 | 9.80 ± 0.54 | 13.0 ± 0.0 |
A feeding trial was conducted in Marine Science Institute of Jeju National University (Jeju, Korea). Olive flounder in growing stage were provided from a local aquafarm. They were acclimated for 2 weeks while feeding the commercial diet. Then, fish (mean body weight, 153 ± 2 g) were randomly selected and distributed into circular tanks (210 L, 25 fish per tank). Twelve tanks were used for quadruplicate groups per dietary treatment. Each tank in the system was designed to receive sand-filtered seawater at 5 L/min flow rate and aerated by air stones. Photoperiod was scheduled for 12:12 h light/dark by fluorescent light. Fish in each tank were fed the diets until satiation (twice a day, 08:30 and 17:30 h) for 12 weeks. The average water temperature during the feeding trial was 22.0 ± 1.8°C. Growth measurements were carried out every two weeks during the feeding trial.
Fish were starved 24 h before the sample collection. Fish weight in each tank was measured to calculate growth results. Blood samples were taken from three fish in each tank after anesthetizing in 200 mg/L 2‐phenoxyethanol (Sigma-Aldrich, St. Louis, MO, USA) solution. Blood was withdrawn with or without heparinized syringes to separate plasma or serum by centrifuging at 5,000×g for 10 min. Then, samples were stored at −70°C before the analyses of hematological and immunological parameters.
Hematological parameters were analyzed using an automated blood analyzer (SLIM, SEAC, Florence, Italy). Hematocrit was estimated through the micro hematocrit technique (VS-12000, Vision Scientific, Daejeon, Korea). Immunological parameters, such as nitro blue tetrazolium assay according to Anderson & Siwicki (1995), myeloperoxidase activity according to Quade & Roth (1997), lysozyme activity according to Khosravi et al. (2015), anti-protease activity according to Ellis (1990), immunoglobulin (Ig) level according to Siwicki & Anderson (1993) and superoxide dismutase activity using an assay kit (19160, Sigma-Aldrich) were analyzed.
Randomly selected fifteen fish from each tank were intraperitoneally injected with pathogenic agent (E. tarda) after the feeding trial. The culture condition of the pathogen was explained by Lee et al. (2020). The injected dose was 1 × 104 CFU/mL which was estimated in a preliminary test with similar sized fish. The injected fish were distributed into nine 125 L acrylic tanks in triplicate groups per diet treatment and mortality was recorded for 15 days. After 15 days of the challenge test, blood was collected from three survived fish per each tank. The collected blood was then separated for plasma and serum samples following aforementioned method.
Results
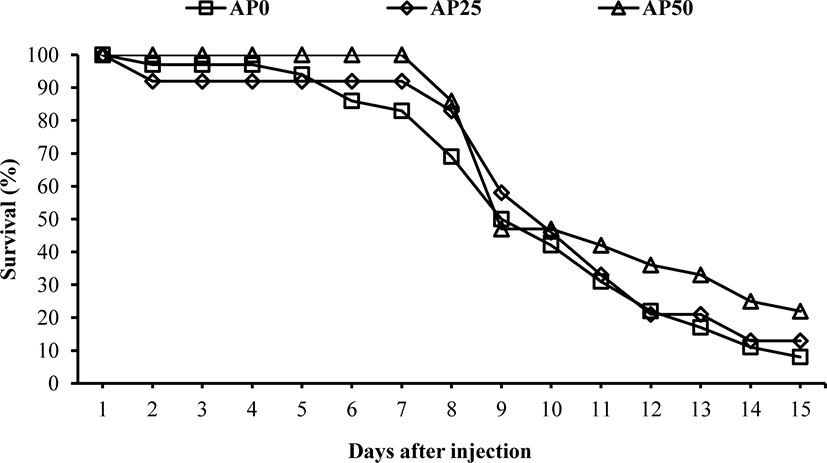
The result of challenge test with E. tarda is shown in Fig. 1. First mortality was recorded in AP0 and AP25 groups on the second day after the injection challenge. In AP50 group, first mortality was observed on the 8th day. The mortality was drastically increased after the 8th day. At the end of the challenge test, fish fed AP50 diet showed significantly higher survival than fish fed AP0 and AP25 diets. Immunological and antioxidant status (Table 2) were not significantly affected before the challenge test. However, after the bacterial challenge, lysozyme, Ig and anti-protease were significantly increased in fish fed diets containing SW1-1 compared to those of fish fed AP0 diet. Hematological parameters showed no significant changes among the groups before the challenge test (Table 3). After the challenge test, significantly lower glucose level was observed in fish fed AP50 diet compared to AP0 group. However, other parameters were not significantly affected. Growth performance and feed utilization of olive flounder fed the experimental diets were not significantly different among the treatments (Table 4). At the end of the feeding trial, numerically increased survival was noticed in AP25 (85.0%) and AP50 (86.7%) groups compared to AP0 group (73.3%) during the feeding trial even though it was not significant.
Discussion
Innate immune parameters of fish were not significantly affected by dietary SW1-1 supplementation after the feeding trial. However, those parameters (lysozyme, Ig and anti-protease) of fish fed AP25 and AP50 diets were significantly enhanced upon the pathogen challenge. In a previous study, respiratory burst and lysozyme activities of fish were not improved by B. polyfermenticus and B. licheniformis after 8 weeks of a feeding trial (Jeong et al., 2006). Cha et al. (2013) observed significantly improved respiratory burst activity in olive flounder fed diets containing B. pumilus and B. subtilis before and after a S. iniae challenge while the innate immunity was not significantly improved by B. licheniformis. Immune-enhancing effects might also depend on the type of Bacillus species (Telli et al., 2014). Dietary Bacillus species is known to upregulate the expression of immune-related genes in olive flounder (Hasan et al., 2019; Nguafack et al., 2020). Additional studies are necessary to elucidate the effects of dietary SW1-1 on the gene expression of the innate immune parameters in olive flounder.
Disease resistance of olive flounder was significantly improved by AP50 diet. Similarly, several Bacillus species were able to enhance the disease resistance of olive flounder (Hasan et al., 2019; Lee et al., 2020; Nguafack et al., 2020). Lee et al. (2020) reported that the improvement in the disease resistance of olive flounder could be attributed to high lysozyme and anti-protease activities followed by B. subtilis administration through the diets. Hasan et al. (2019) suggested that the improved disease resistance of olive flounder fed Bacillus species might be a result of increased immunity and accelerated anti-inflammatory cytokine (IL-10) expression in liver, kidney, spleen, and gill. Nguafack et al. (2020) attributed these positive effects on the disease resistance of Bacillus-fed fish to an increased expression of pro-inflammatory cytokines and an enhancement of other innate immune parameters. In the present study, we did not observe improved immunity right after the feeding trial although immune parameters and disease resistance were significantly higher in SW1-1 groups after the challenge with E. tarda. Therefore, the present result might indicate that the innate immunity of fish is likely to be quickly boosted when fishes face to pathogenic environment or a certain stress. Plasma glucose level of olive flounder was significantly lower in AP50 group compared to AP0 group after the challenge test. Blood glucose level in fish is usually increased in response to stresses (Nakano et al., 2014). Plasma glucose level could also be reduced under feed deprivation conditions (Polakof et al., 2011). In the present study, fish were starved during the challenge test. Therefore, lower blood glucose levels after the challenge test compared to those after the feeding trial might be attributed to food deprivation. Particularly, AP25 and AP50 groups exhibited higher innate immunity than AP0 group during the pathogenic challenge without feeding. Higher immune activity was known to increase metabolic rate of fish (Bonneaud et al., 2016; Skinner et al., 2010). Thus, the decreased plasma glucose levels seem to be due to the improved innate immunity by the dietary supplementation of SW1-1 leading to an increased metabolic rate of the fish in this study.
Growth performance and feed utilization of olive flounder was not significantly affected by dietary SW1-1 supplementation as demonstrated by the results of the feeding trial. In previous studies, growth performance of olive flounder was improved by several Bacillus species including B. subtilis (Cha et al., 2013; Lee et al., 2020) and Bacillussp. SJ-10 (Hasan et al., 2018, 2019; Jang et al., 2021; Nguafack et al., 2020). Several studies have reported that growth performance of olive flounder was not affected by dietary supplementation with Bacillus species including B. polyfermenticus, B. licheniformis, and B. pumilus (Cha et al., 2013; Jeong et al., 2006; Niu et al., 2021). Therefore, growth promotion might be associated with the types of Bacillus species. Telli et al. (2014) observed that a stocking density could be a factor that can affect the growth of Nile tilapia (Oreochromis niloticus) by dietary supplementation of B. subtilis. Giri et al. (2013) reported that the growth performance of Labeo rohita was significantly affected by different doses of L. plantarum in diet suggesting that the effects of probiotics may also be dependent on their inclusion levels. Therefore, the discrepancy in the growth results might be attributed to the fish stocking density and dietary probiotic levels or types. Further studies need to be conducted to optimize the level of Bacillus SW1-1 in olive flounder diet.
