Introduction
Norovirus accounts for more than 90% of non-bacterial enteritis and is the most common cause of enteritis, regardless of age or sex (Fankhauser et al., 2002; Lee &Yoon, 2021). The infectious dose of norovirus is low, and a 50% infectious dose has been reported as 18 viruses (Teunis et al., 2008). This virus is mainly transmitted through the fecal-oral route, and water contaminated with norovirus is the leading cause of infection due to poor sewage treatment. However, food can also be a significant vector of norovirus food poisoning accidents, contaminated water for foodborne illness, or secondary infection through food industry workers.
Foodborne illness caused by norovirus has been identified as a significant cause of foodborne illness in Korea and Europe, and the United States (Lee &Yoon, 2021; Møretrø et al., 2021). As a result of the survey of foodborne illness incidence in Korea over the last 10 years, the number of foodborne illnesses with norovirus was 771. The incidence was the highest except for cases of unknown cause. The number of patients was 24,780. The causative agent caused many patients after pathogenic Escherichia coli, which causes intestinal disease and sepsis (Cavani et al., 2022; MFDS, 2021).
Among foods, filter-feeding bivalve shellfish such as oysters and mussels accumulate norovirus in the contaminated water and are considered a significant source of contamination. Oysters, which are occasionally eaten raw, are an important vector for norovirus infection. Quantitative microbial risk assessment is performed to prepare scientific evidence for food safety, and the risk potential of foodborne pathogens is evaluated (Ha et al., 2020; Lee et al., 2019). Accordingly, this study evaluated the risk of norovirus foodborne illness by raw oyster consumption.
Materials and Methods
Oyster samples (n = 156) were purchased from supermarkets in Korea. After separating the mid-gut gland inside the oyster, the surrounding tissue was removed, and 3 g of the mid-gut gland was collected in a 50-mL tube. Three milliliters of proteinase K were added into the tube, and this mixture was homogenized entirely 3 g with a homogenizer. This homogenate was reacted for 60 min in a shaking incubator at 230×g and 37°C. After reacting for 15 min in a water bath at 60 ± 2°C, the homogenate was centrifuged at 4,000×g for 5 min. The supernatant (1.5 mL) was then centrifuged at 4,000×g for 2 min, and the final supernatant was used for RNA extraction.
RNeasy mini kit (Qiagen, Valencia, CA, USA) was used to extract viral RNA according to the manufacturer’s instructions (Seo et al., 2021). AVL buffer (1,120 µL) was mixed with 280 µL of the final supernatant, allowed to stand at room temperature for 10 min, and then lightly centrifuged using a microcentrifuge. After mixing 1,120 µL of 95%–100% ethanol in the mixed solution, it was lightly centrifuged. Six hundred thirty microliters of the mixed solution were transferred to a small centrifugal column, and the column was centrifuged at 6,000×g for 1 min. The column was transferred to a new extraction tube, and 630 µL of the mixed solution was then added to the column and centrifuged. The small-volume centrifugal column was centrifuged to a new extraction tube, and 500 µL of AW1 buffer was added to the column, followed by centrifuging at 6,000×g for 1 min. This column was then transferred to a new 1.5-mL extraction tube. Five hundred microliter of AW2 buffer was added to the column, and it was centrifuged at 20,000×g for 3 min. The column was transferred to a new 1.5-mL extraction tube, and 60 µL of AVE buffer was added. The column was centrifuged at 6,000×g for 1 min to be used as a template for PCR. The PCR conditions were 30 min at 45°C and 10 min at 95°C, followed by 45 cycles at 95°C for 15 s and 60 s at 56°C once, and then the amplification curve was confirmed using a real-time PCR system (Applied Biosystems, Waltham, MA, USA) (Jo et al., 2021; Kang et al., 2021). The sequences of primer and probe and composition of the PCR reaction solution of norovirus and were shown in Tables 1 and 2.
| Genotype | Sequence (5’→3’) | Reference |
|---|---|---|
| GI | Forward: CGY TGG ATG CGN TTY CAT GA | MFDS (2020) |
| Reverse: CTT AGA CGC CAT CAT CAT TYA C | ||
| Probe: FAM-AGA TYG CGA TCY CCT GTC CA-TAMRA | ||
| GII | Forward 1: AIC CIA TGT TYA GIT GGA TGA G | MFDS (2020) |
| Forward 2: AGT CAA TGT TTA GGT GGA TGA G | ||
| Reverse: TCG ACG CCA TCT TCA TTC ACA | ||
| Probe: VIC-CAC RTG GGA GGG CGA TCG CAA TC-TAMRA |
After grinding 25 g of an oyster sample in a blender for 1 min, 2 g was placed in a 50-mL centrifuge tube. Twenty-five gram portions of oyster samples were inoculated with 300 µL of murine norovirus (MNV) of 8.9 Log PFU/mL and vortexed for 5 min. Due to the difficulty of a human norovirus cell culture system, MNV was used as a human norovirus surrogate (Cannon et al., 2006; Zonta et al., 2016). The virus plaque counts in the samples were enumerated during incubation at 4°C, 15°C, and 25°C for 0, 1, 2, 3, 5, and 7 days. Eighteen milliliters of glycine buffer (pH 9.5; 0.1 M glycine, 0.3 M NaCl) was added to the oyster samples, and each sample was stored at 4°C, 15°C, and 25°C. After vortexing for 5 min at 21°C, the suspension was centrifuged at 10,000×g, for 15 min at 4°C to obtain the supernatant. Twenty milliliters of 16% polyethylene glycol 8,000 containing 0.25 M NaCl was added to the supernatant, and 60 oscillations/min constant rocking was performed at room temperature for 1 h. Thereafter, the suspension was centrifuged at 10,000×g and 4°C for 10 min, and the supernatant was discarded. The pellet was then resuspended in 1 mL of phosphate-buffered saline. The number of MNV was quantified by a plaque assay. The plaque counts (Log PFU/g) of MNV were fitted with the Baranyi model as follows to develop a primary model (Baranyi & Roberts, 1994).
where Nt is the viral titers at time t, N0 and Nmax are the initial and final viral titers, respectively, and h0 is a parameter defining the initial physiological state of the virus. DR (Log PFU/g/h) means the death rate of the norovirus, and At is the adjustment function, which denotes the physiological status of viral titers when defining the shoulder period (Baranyi & Roberts, 1994). To evaluate the temperature effect on the kinetic parameters, DR values were fitted with a secondary model as follows as a function of the temperature.
In order to validate the performance of the developed models, the observed values of MNV obtained at 10°C and 20°C were compared to the predicted values obtained from the developed models. Root mean square error (RMSE), bias factor (Bf), and accuracy factor (Af) were calculated between observed and predicted values.
The temperature and time data were collected during transport and storage, and the data were taken into the developed models, and the predictive models described plaque counts of MNV.
To obtain the consumption ratio and amounts of oyster, the 2018 Korea National Health and Nutrition Examination Survey (KNHANES) raw data conducted by Korea Disease Control and Prevention Agency (KDCA) were extracted using SAS® version 9.4 (SAS Institute, Cary, NC, USA), and a redundancy test was performed on the extracted data.
To evaluate the risk according to the intake dose of norovirus, dose-response models were searched through literatures, and the most appropriate model was selected.
A simulation model was prepared with the norovirus contamination level in raw oysters, developed predictive models, temperatures and time for transport and storage, ratio and amounts of consumption, and a dose-response model to estimate norovirus risk through raw oyster consumption in an Excel spreadsheet. The simulation was analyzed using @RISK with 10,000 iterations. In addition, the variables that had the most significant influence on the risk were identified.
This study used the quantitative microbial risk assessment results of norovirus foodborne illness by raw oyster consumption to estimate an actual number of foodborne illness cases. Since the risk calculated through risk assessment is the probability of occurrence of foodborne illness, ‘final risk × number of Korean populations’ was calculated to estimate the number of patients with foodborne illness. The calculated risk is based on the two assumptions that the same response appears to all and that one person suffers from foodborne illness only once. The 2021 statistics from the Ministry of the Interior and Safety were used for Korean populations (KOSIS, 2021). The Cost-Of-Illness (COI) method was used to estimate socio-economic costs due to foodborne illness, and cost items are largely divided into direct costs and indirect costs (Shin et al., 2010; Table 3). As for direct costs, direct medical costs including outpatient care, inpatient care, medication, and direct non-medical cost including transportation and caregiving costs were calculated. For outpatient (inpatient) care cost, outpatient (inpatient) cost per person per day, and the average number of outpatients (inpatient) visit days were used. The medical cost was calculated by multiplying the estimated number of patients by the medical cost per person (Shin et al., 2010). Statistical data from the Korea Health Panel Study (KHPS) were used for transportation costs per person. The final transportation cost was calculated assuming that one guardian was accompanying them (KHPS, 2019). For care costs, the daily caregiver fee suggested by the Seoul Caregiver Association was used. As the indirect cost, the lost productivity cost for outpatients and inpatients was calculated. The lost productivity cost for outpatients was calculated using the average wage data of employment and labor statistics, assuming that the outpatient visit time is 1/3 of the daily working hours (Lee et al., 2016; MOEL, 2021). The lost productivity cost for inpatients was calculated using average wage data from employment and labor statistics to calculate the cost of lost income for hospitalization days (MOEL, 2021). All expenses in Korean currency were converted to U.S. dollars at 1,146 won = 1 dollar in the exchange rate as of August 6, 2021.
| Type of cost | Categories | Data source | |
|---|---|---|---|
| Direct cost | Direct medical cost | Outpatient care cost | KOSIS (2021); HIRA (2020) |
| Inpatient care cost | |||
| Medication cost | Shin et al. (2010) | ||
| Direct non-medical cost | Transportation cost | KHPS (2019) | |
| Care-giving cost | Seoul Caregiver Association (2021) | ||
| Indirect cost | Lost productivity cost | MOEL (2021) | |
Results and Discussion
One of 156 oyster samples was positive for norovirus, and the number of the positive sample was used as an input variable for the Beta distribution. In other studies that performed a risk assessment for norovirus, 5 to 138 samples were collected to estimate prevalence and initial contamination (IC) levels, and investigated the levels of norovirus contamination, depending on the studies (Bouwknegt et al., 2015; Torok et al., 2019). However, in our study, 156 samples were analyzed. Thus, the number of samples could be considered sufficient for the risk assessment of oyster consumption in Korea. As a result of estimating the initial level of norovirus contamination in oysters by the above method, the average initial level of contamination was –5.3 Log PFU/g.
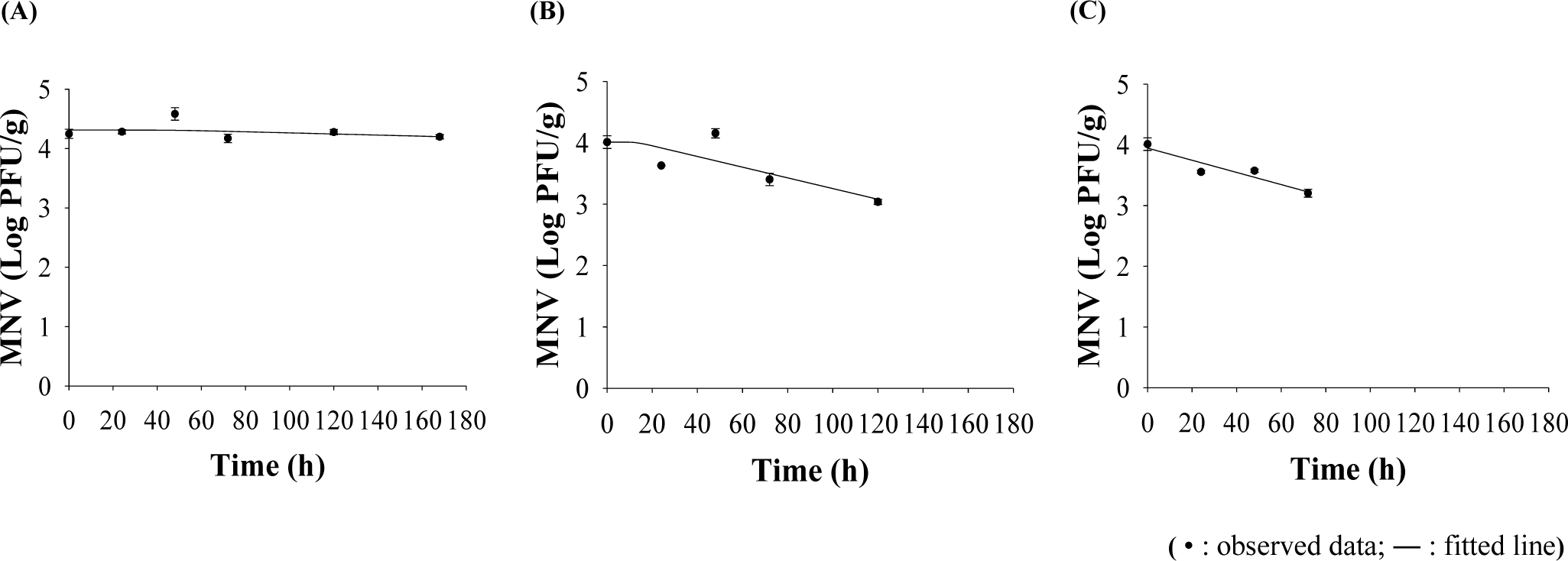
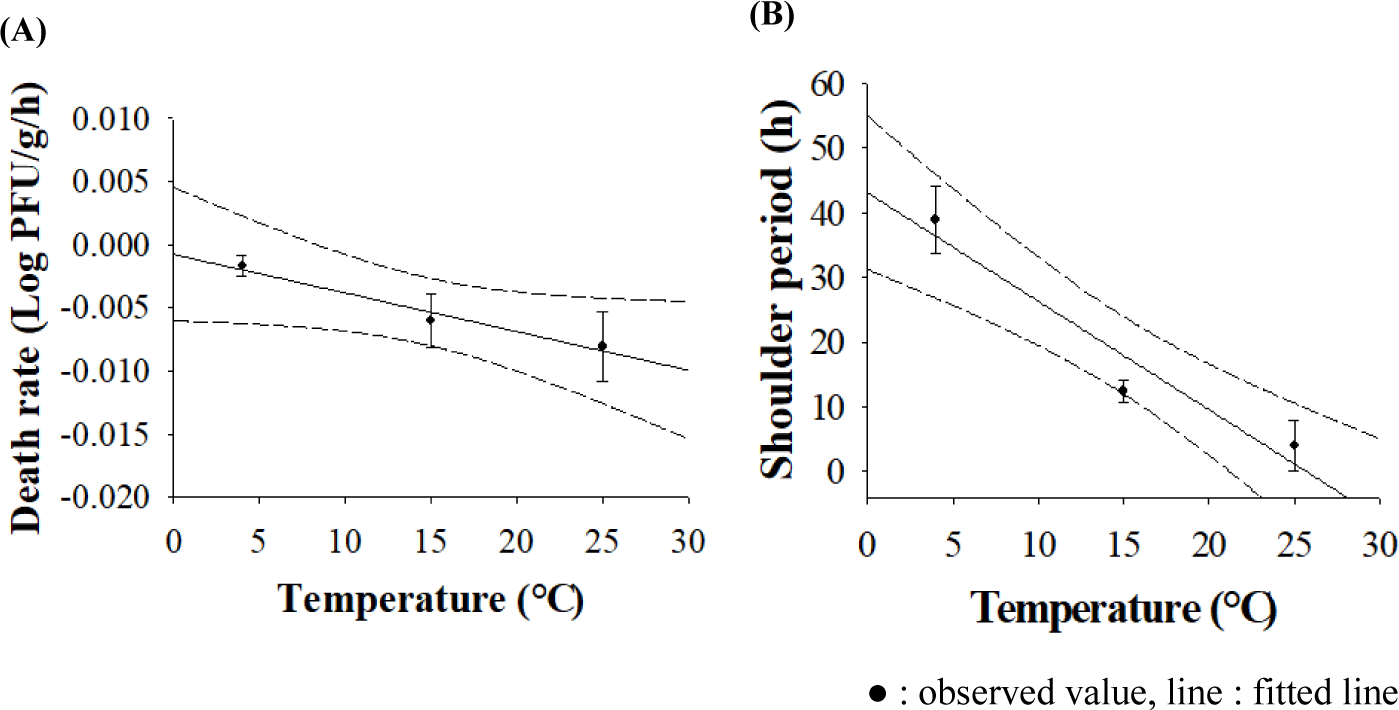
The R2 values of the developed primary models, describing the death, were 0.44 at 15°C and 0.50 at 25°C. The MNV concentration was not changed at 4°C. They indicate that the developed models were appropriate for describing changes in norovirus plaque counts in the oyster. At 4°C, the number of viruses did not change, but at 15°C and 25°C, the number of viruses decreased rapidly at –6.0 × 10−3 and –8.1 × 10−3 Log PFU/g/h overtime after 12 and 4 h of shoulder period, respectively (Fig. 1 and Table 4). Developed secondary models were DR = –0.0008 – 0.0003 × T (R2 = 0.436) and shoulder period = 43.0332 – 1.6754 × T (R2 = 0.821). They were developed to describe the changes in death rate and shoulder period by temperature (Fig. 2). Although the R2 value of the secondary model for the death rate was low, RMSE values from the validation showed 0.070 and 0.046 for 10°C and 20°C, respectively, and even Bf (1.00) and Af (1.02) were low, regardless of temperature. Thus, the developed models were appropriate to changes in the virus numbers by changing temperature and time.
| Parameter | Temperature (°C) | ||
|---|---|---|---|
| 4 | 15 | 25 | |
| Death rate (Log PFU/g/h) | –1.7 × 10−3 | –6.0 × 10−3 | –8.1 × 10−3 |
| Shoulder period (h) | 39 | 12 | 4 |
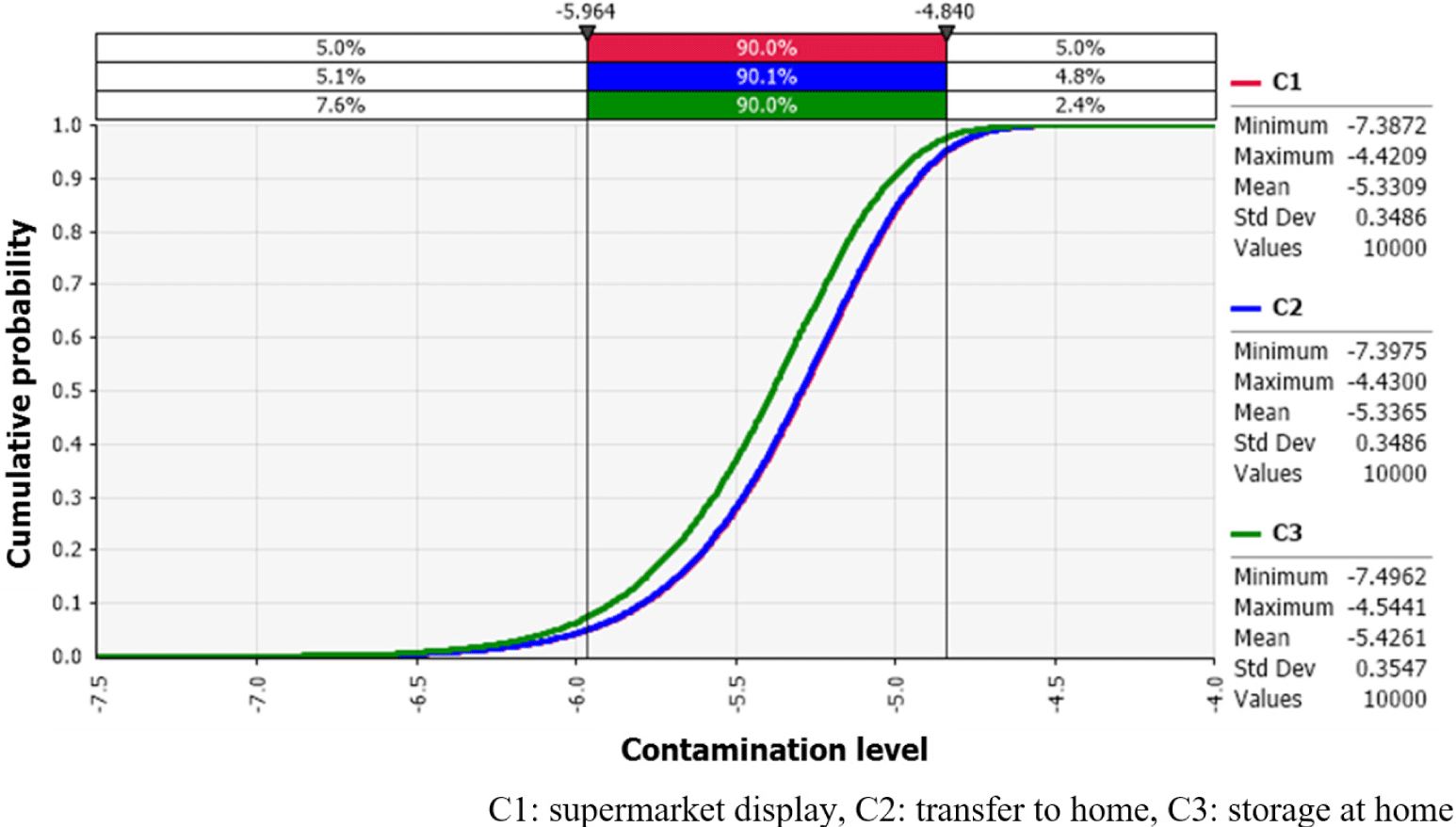
The displayed temperature at supermarkets was at 2°C–4°C, and thus, the appropriate probabilistic distribution was the Uniform distribution [Uniform (2, 4)]. The display time at supermarkets was less than 1 day, and thus, the appropriate probabilistic distribution was the Uniform distribution [Uniform (0, 24)]. Reference literature was used to obtain data for the time and temperature during the transit period from supermarket to home (Jung, 2011). The transit time was 0.325–1.643 h, and the temperature was 10°C–25°C. Thus, these values were fitted with the Uniform distribution [Uniform (0.325, 1.643)] for time and the Pert distribution [Pert (10, 18, 25)] for the temperature. A study by Jeong et al. (2015) showed that the storage period of oysters at home was investigated as 96 h at maximum, but some consumers ate oysters right after they arrived at home. Hence, the Uniform distribution [Uniform (0, 96)] was used for the home storage time. For storage temperature at home, the LogLogistic distribution [LogLogistic{–29.283, 33.227, 26.666, Truncate (–5, 20)}] suggested a study by Lee et al. (2015) was cited. The developed predictive models predicted the changes in norovirus concentrations in accordance with these probabilistic distributions for temperature and time. The change in the distribution of the norovirus contamination level according to the distribution stage is shown in Fig. 3. As the IC goes through the distribution stages of supermarket display (C1)–transfer to home (C2)–storage at home (C3), the average estimated contamination level is –5.3 Log PFU/g to –5.4 Log PFU/g. When transferring from the supermarket to the home, the temperature during transfer was room temperature, and the transfer time did not exceed 2 h. Considering that the shoulder period was 4 h at 25°C, it can be inferred that norovirus concentration in oysters was not changed during the transit time to the home. The developed predictive models showed that the number of viruses tended to be maintained at 4°C, and the number of the viruses was rapidly decreased as the temperature increased. The shelf life of raw oysters is generally about 1–3 days. As a result of the distribution environment survey in this study, it was confirmed that oysters are distributed at refrigerated temperatures (Jeong et al., 2015). Therefore, the probability of oysters being exposed to high temperatures during distribution and storage is low. Hence, it is considered that the number of norovirus at the time of consumption by consumers is not significantly different from the IC level of oysters.
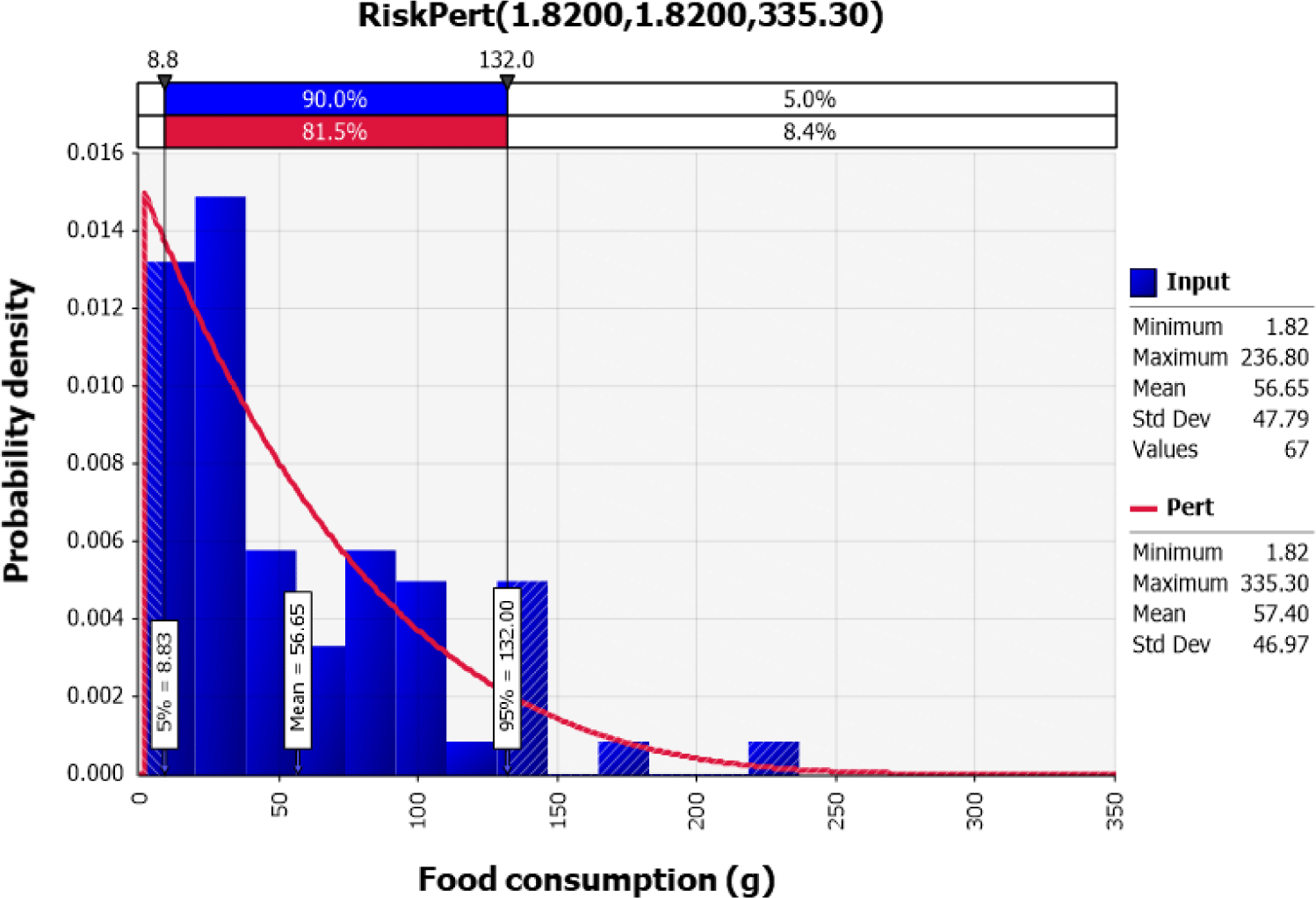
Of the 7,064 total respondents to the KNHANES conducted in 2018, 69 people responded that they ate raw oysters, and the ratio of those who consumed raw oysters was 0.98%. Raw data for consumption amounts of the 0.98% respondents were analyzed with @RISK, and the Pert distribution [Pert (1.8200, 1.8200, 335.30)] was appropriate, which showed that the average amount of consumption was 56.65 g (Fig. 4).
The 1F1 hypergeometric dose-response model [P = 1 – (1 + ηCV)−r] (η, 2.55 × 10−3; CV, concentration × volume = dose; r, 0.086) developed by Teunis et al. (2008) was used to calculate a dose-response of norovirus.
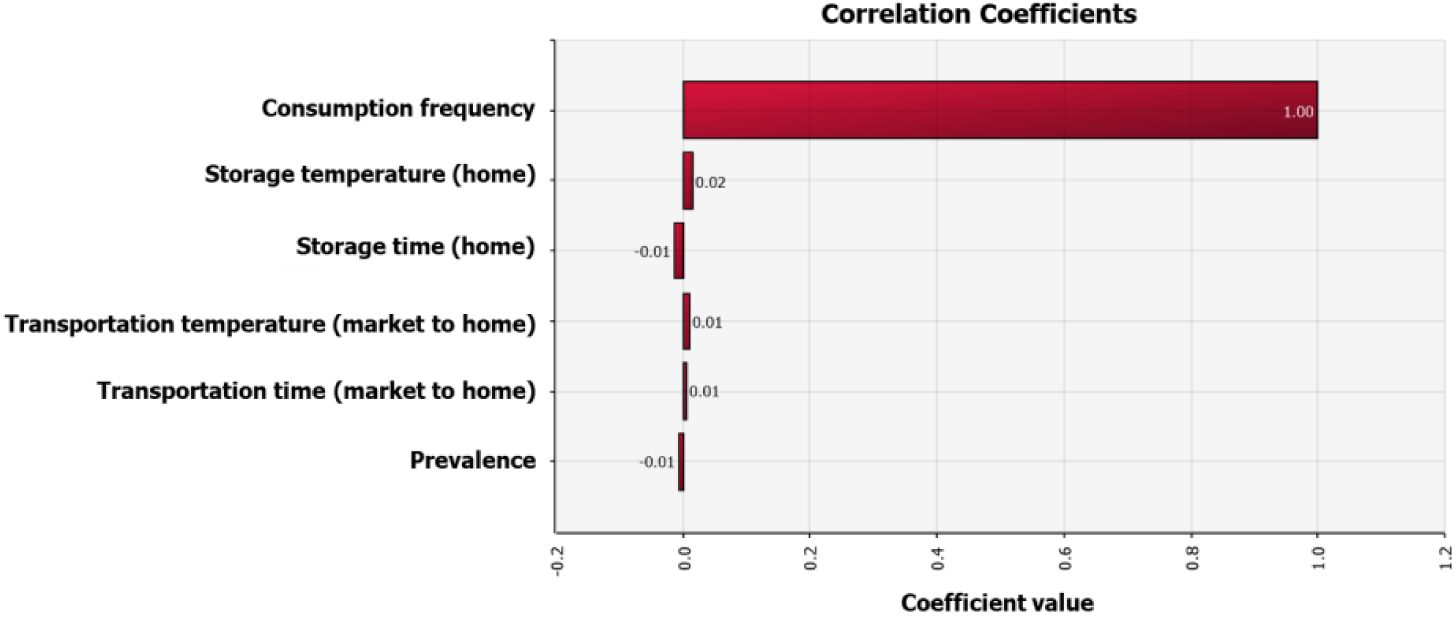
To estimate the probability of occurrence of norovirus foodborne illness in one person per day when oysters are consumed, a simulation model was developed by composing a scenario such as Table 5 in an Excel spreadsheet linked with @RISK based on the above results. As a result of estimating the risk of norovirus in oysters through simulation using the @RISK fitting program, the probability of occurrence of foodborne illness due to norovirus in one person per day when oysters are consumed is 5.90 × 10−10 (minimum 0, maximum 3.85 × 10−7). As a result of performing quantitative microbial risk assessment (QMRA) using data contaminated with norovirus in 184 out of 2,234 oyster samples, the probability of foodborne illness due to norovirus in one person per day when oysters are consumed was 1.41 × 10−2 (Kim & Kim, 2017). This study was judged to be more dangerous than the risk of our study because the risk assessment was conducted under the assumption that consumers immediately consume oysters contaminated with norovirus without exposure to the distribution environment using high IC level data. According to the contamination level results in this study, only 1 of 156 oyster samples was positive for norovirus. Therefore, if the number of samples of oysters contaminated with norovirus increases, the risk may increase. As a result of analyzing the correlation between variables and risk, the consumption ratio had the most significant effect on the risk (Fig. 5).
| Input model | Unit | Variable | Formula | Reference |
|---|---|---|---|---|
| Product | ||||
| Norovirus prevalence | PR | = RiskBeta(2, 156) | This research; Vose (1997) | |
| Norovirus concentration | GC/g | C | = –LN(1 – PR) / 2 g | Sanna et al. (2004) |
| Initial contamination level | PFU/g | IC | = 1 / 1,000 × C | Jensen et al. (2018) |
| Log PFU/g | LogIC | = Log(IC) | ||
| Market display | ||||
| Display time | h | Timemark-dis | = RiskUniform(0, 24) | Personal communication |
| Food temperature during display | °C | Tempmark-dis | = RiskUniform(2, 4) | Personal communication |
| Death | ||||
| h0 | = Fixed 0.001 | This research | ||
| Log PFU/g | Y0 | = average(Y0i) Fixed 4.1 | This research | |
| Log PFU/g | Yend | = average(Yendi) Fixed 3.3 | This research | |
| LN(q) | = LN(1 / (EXP(h0) – 1)) | This research | ||
| Death rate | Log PFU/g/h | DRmark-dis | = –0.0008 – 0.0003 × Tempmark-dis | This research |
| Norovirus death | Log PFU/g | C1 | = LogIC + 1 / (1 + EXP(–LN(q))) × (1 – (10−lY0-Yendl / LN(10))) × DRmark-dis × Timemark-dis | This research |
| Transportation (vehicle) | ||||
| Transportation time | h | Timeveh | = RiskUniform(0.325, 1.643) | Jung (2011) |
| Food temperature during transportation | °C | Tempveh | = RiskPert(10, 18, 25) | Jung (2011) |
| Death | ||||
| h0 | = Fixed 0.001 | = Fixed 0.001 | ||
| Log PFU/g | Y0 | = average(Y0i) Fixed 4.1 | This research | |
| Log PFU/g | Yend | = average(Yendi) Fixed 3.3 | This research | |
| LN(q) | = LN(1 / (EXP(h0) – 1)) | This research | ||
| Death rate | Log PFU/g/h | DRveh | = –0.0008 – 0.0003 × Tempveh | This research |
| Norovirus death | Log PFU/g | C2 | = C1 + 1 / (1 + EXP(–LN(q))) × (1 – (10−lY0–Yendl/ LN(10))) × DRveh × Timeveh | This research |
| Home storage | ||||
| Storage time | h | Timehome | = RiskUniform(0, 96) | Jeong et al. (2015) |
| Food temperature during storage | °C | Temphome | = RiskLogLogistic(–29.283, 33.227, 26.666, RiskTruncate(–5, 20)) | Lee et al. (2015) |
| Death | ||||
| h0 | = Fixed 0.001 | This research | ||
| Log PFU/g | Y0 | = average(Y0i) Fixed 4.1 | This research | |
| Log PFU/g | Yend | = average(Yendi) Fixed 3.3 | This research | |
| LN(q) | = LN(1 / (EXP(h0) – 1)) | This research | ||
| Death rate | Log PFU/g/h | DRhome | = –0.0008 – 0.0003 × Temphome | This research |
| Norovirus death | Log PFU/g | C3 | = C2 + 1 / (1 + EXP(–LN(q))) × (1 – (10−lY0–Yendl/ LN(10))) × DRhome × Timehome | This research |
| PFU/g | C3PFU/g | = 10C3 | This research | |
| Consumption | ||||
| Daily consumption average amount | g | Consump | = RiskPert(1.8200, 1.8200, 335.30, RiskTruncate(0, 236.8)) | KDCA (2020) |
| Daily consumption ratio | % | ConFre | = Fixed 0.98 | KDCA (2020) |
| CF(0) | = 1 – 0.98 / 100 | KDCA (2020) | ||
| CF(1) | = 0.98 / 100 | KDCA (2020) | ||
| CF | = RiskDiscrete({0, 1}, {CF(0), CF(1)}) | KDCA (2020) | ||
| g | Amount | = IF(CF = 0, 0, Consump) | KDCA (2020) | |
| Dose-response | ||||
| Norovirus amount | PFU/g | CV(dose) | = C3PFU/g× Amount | |
| Parameter | η | = Fixed 2.55 × 10−3 | Teunis et al. (2008) | |
| r | = Fixed 0.086 | Teunis et al. (2008) | ||
| Risk | ||||
| Probability of illness/person/day | Risk | = 1 – (1 + ηCV)−r | Teunis et al. (2008) | |
The final risk of the quantitative microbial risk assessment of norovirus by oyster consumption was estimated to be 5.90 × 10−10/person/day, and the population of South Korea was 51,829,136. Therefore, as a result of estimating the number of patients with norovirus foodborne illness caused by oyster consumption in South Korea, it was 0.03 person occurred per day. Accordingly, the socio-economic cost of norovirus foodborne illness by oyster consumption was estimated to be $14.2 per day and was estimated to be only $5,183 per year (Table 6).
Conclusion
The probability of norovirus foodborne illness caused by oysters in Korea is 5.90 × 10−10/person/day, and it was confirmed that the consumption ratio had the most significant effect on the risk. In addition, the annual socio-economic cost for the norovirus foodborne illness by oyster consumption can be considered low.