Introduction
Goldfish (Carassius auratus L.) is a freshwater fish belonging to family Cyprinidae of order Cypriniformes. Goldfish generally has strong vitality. However, several diseases can cause mortality of goldfish (Groff et al., 1998; Iqbal et al., 1999; Jung & Miyazaki, 1995). Members of the cyprinid herpesviridae family are divided into carp pox herpesvirus (Cyprinid herpesvirus 1, CyHV-1), goldfish hematopoietic necrosis herpesvirus (Cyprinid herpesvirus 2, CyHV-2) and koi herpesvirus (Cyprinid herpesvirus 3, CyHV-3) (Davison et al., 2013; Waltzek et al., 2005). CyHV-2 causes hematopoietic necrosis, resulting in herpesviral hematopoietic necrosis. This disease first occurred in Japan (1992 and 1993), causing severe mortality (up to 100%) of goldfish (Jung & Miyazaki, 1995). Since then, CyHV-2 outbreaks in goldfish have been reported in many countries, including Australia, India, France, Taiwan, UK, and USA (Boitard et al., 2016; Chang et al., 1999; Goodwin et al., 2006; Groff et al., 1998; Jeffery et al., 2007; Sahoo et al., 2016; Stephens et al., 2004). Moreover, CyHV-2 has been detected in prussian carp (Carassius giberio B.) and crucian carp (Carassius carassius L.) from Czech, China, Hungary, and Italy, causing massive mortality in recent years (Daněk et al., 2012; Doszpoly et al., 2011; Fichi et al., 2013; Luo et al., 2013; Wang et al., 2012). The tendency of CyHV-2 spreading globally is significant.
This study describes two cases of CyHV-2 detection in Korea. Additionally, artificial infection experiment of CyHV-2 was carried out to confirm that the cause of goldfish mortality was CyHV-2.
Materials and Methods
Case I: In April 2014, mortalities of goldfish (9.9 ± 0.4 cm, 17 ± 0.7 g) occurred at a local aquarium in Korea. Fish were maintained in a 4,000 L tank with recirculating water system. Water temperature, pH and dissolved oxygen in the tank were 21°C–22°C, 7.57 and 7.11, respectively. The estimated mortality was about 33% (230 out of 700 fish) in this incident. Affected fish showed reduced feed intake, lethargy and atypical swimming at edges of tank. Diseased fish showed pale gills, severely enlarged and softened spleen and kidney and red liver (Fig. 1A). After confirming CyHV-2 infection, all survived goldfish were sacrificed. The facility was then disinfected.
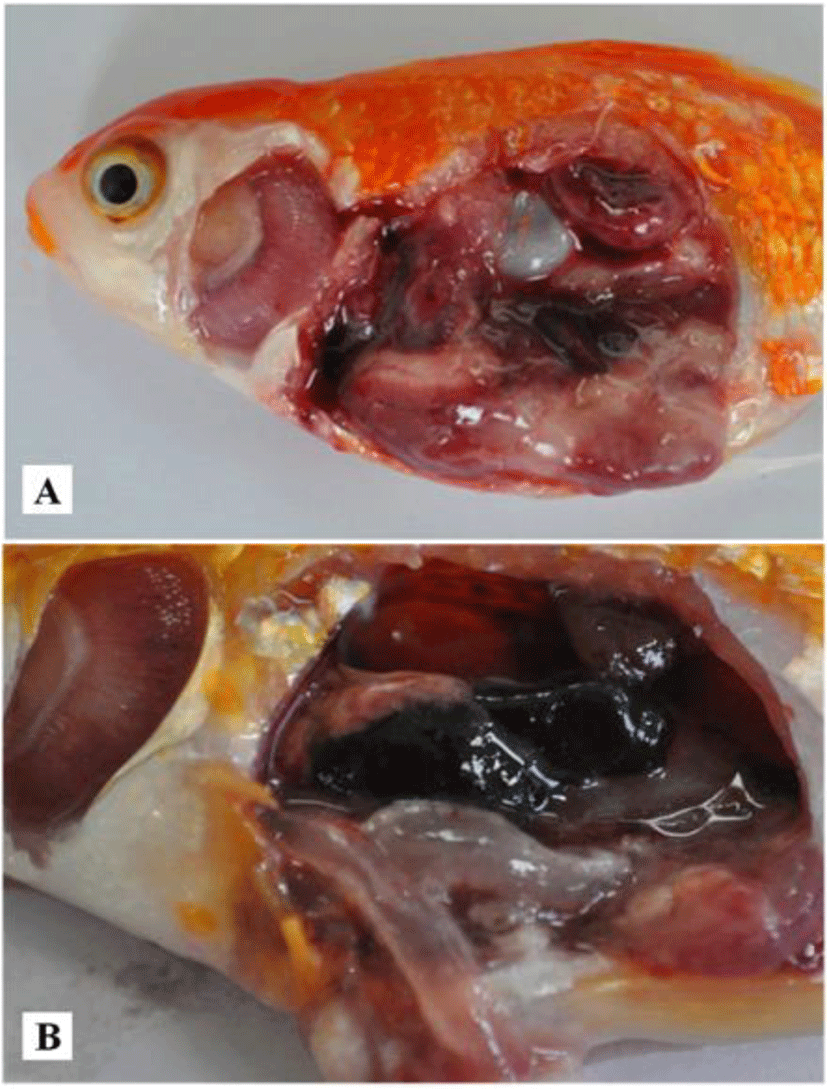
Case II: In June 2014, goldfish (3.6 ± 0.4 cm, 4.6 ± 1.1 g) were purchased from an online market. Fish started to die from 7 days after arrival. Fish showed lethargy without clinical signs. Internal organs showed gill pallor and enlarged spleen and kidney. Thinned intestinal wall was filled with transparent fluid (Fig. 1B).
Goldfish gills were examined directly under microscope to identify possible parasite infections. Aseptically collected kidney from affected fish were inoculated in brain heart infusion agar (BHIA, 0.5%) (BD Difco, Sparks, MD, USA), thiosulfate citrate bile sucrose agar and salmonella shigella agar and incubated at 23°C for 2 days to identify pathogenic bacteria.
The virus was prepared from kidney and spleens of affected goldfish which obtained from the above described online local fish market. The organs were added to 50-fold volumes of Dulbecco’s Minimum Essential Medium (DMEM) (Gibco, Grand Island, NY, USA) and homogenized using Polytron PT 1200 E homogenizer (Kinematica, Littau, Switzerland) on ice until dissolved completely. Homogenate was centrifuged at 737×g for 30 min at 4°C. Supernatant was filtered through a 0.45-μm Acro-disc® syringe filter (Pall, Ann Arbor, MI, USA) and considered as original virus dose and stored at –80°C until use.
For histopathological observations, dissected tissues of infected goldfish gills, heart, intestine, kidney, liver and spleen were fixed in 10% phosphate-buffered formalin solution and rinsed in tap water. Fixed tissues were serially dehydrated using ethanol and were embedded in paraffin wax using standard protocols. Sections were cut at 4 μm using Shandon AS 325 manual micro-tome (Shandon, Runcorn, UK), mounted on glass slides, and air dried overnight before staining with hematoxylin and eosin (H&E). Sections were analyzed under light microscopy and digital images were captured using NIS-Elements F software (version 3.22).
To confirm the CyHV-2 infection, PCR was conducted by amplifying DNA polymerase gene of CyHV-2 using a primer set (F 5’ CGGAATTCTAGAYTTYGCNWSNYTNTAYCC 3’ Y = C, T; N = ACGT; W = AT; S = CG; R = AG and R 5’ CCCGAATTCAGATCTCNGTRTCNCCRTA 3’) (Goodwin et al., 2006). The genomic DNA was isolated from the kidney and spleen from goldfish using an AccuPrep® Genomic DNA extraction kit (Bioneer, Daejeon, Korea) according to the manufacturer’s instructions. Extracted genomic DNA was dissolved in 100 μL of diethyl pyrocarbonate (DEPC)-treated water and stored at –20°C until use. The PCR reactions were performed using AccuPower® PCR PreMix (Bioneer) in a final reaction volume of 20 μL, containing 1 μL of each primer (10 μM), 13 μL of DEPC-treated water and 5 μL of the template (genomic DNA) by MyGenie 32 Thermal Block (Bioneer). PCR amplification conditions were as follows: 94°C for 1 min, followed by 30 cycles of 94°C for 30 s, 45°C for 30 s and 72°C for 3 min (Goodwin et al., 2006).
The amplified PCR products of virus were analyzed in 1.5% agarose gels containing ethidium bromide and visualized under UV light. The PCR products were resolved from the agarose gel, and purified using AccuPrep® Gel purification kit (Bioneer) and cloned into pGEM®-T easy vector for sequencing (Bioneer) according to manufacturer’s protocol. The resulted sequence was used to search for similarity using BLASTN of NCBI.
For experimental infections, a total number of 60 healthy goldfish (10.4 ± 1.1 cm, 16.9 ± 0.5 g) were obtained from local fish farm in Suncheon, Jeollanam-do, Korea. Five randomly selected fish were confirmed to be free from pathogens (the absence of parasites under microscopic observation, absence of bacteria on BHIA and no amplified CyHV-2 DNA polymerase gene product by PCR). Fish were maintained 2 weeks in quarantine room before challenge test.
Fish were intra-peritoneally (i.p.) injected with kidney and spleen filtrate (100 μL/fish) at the original concentration (1:50 w/v diluted with DMEM) and at 10-, 100-, and 1,000- fold dilutions. Phosphate buffered saline (100 μL/fish) was i.p. injected into control fish. Each group (10 fish per group) was maintained at 21°C for 30 days in the aquaria containing 30 L of fresh water. All dead fish in the CyHV-2 infection group were collected for the PCR assay of the pathogen and histological observation of the infected tissues.
Results and Discussion
In April 2014 sample (Case I), a few Dactylogyrus sp. in affected gills were observed. In addition, a bacterium was isolated from two out of three fish examined shared high homology with 16S rRNA sequences of Aeromonas spp.: 97.1% with Aeromonas sp. (accession number: HQ436040.1), 96.6% with Aeromonashydrophilia (accession number: HQ731685.1) and 96.1% with Aeromonas veronii (accession number: FJ940850.1). Lethal dose 50 from the infection experiment using the isolated bacterium was calculated to be 5 × 105 CFU/fish (data not shown). No bacterium was grown from ‘Case II’ of the June 2014 sample.
In both natural disease cases in this study, clinical signs of gill pallor and enlargement of spleen and kidney were similar to those reported previously (Groff et al., 1998; Jeffery et al., 2007; Jung & Miyazaki, 1995). Red liver was shown only in ‘Case I’. It might be due to mixed infection by Aeromonas sp. Several Aeromonas spp. can cause goldfish mortality (Iqbal et al., 1999; Wiklund & Dalsgaard, 1998). Moreover, CyHV-2 detection associated with A. hydrophila or Aeromona sobria, similar to ‘Case I’, has been reported in crucian carp in Italy and goldfish in India (Claudio et al., 2017; Fichi et al., 2013; Sahoo et al., 2016). Further characterization, identification and the effect of mixed infection of isolated bacteria with CyHV-2 on goldfish mortality are necessary.
To confirm CyHV-2 infection, CyHV-2 polymerase gene (expected size, 520 bp) was amplified from kidney and spleen pools of all naturally infected fish (3 fish from ‘Case I’ and 5 fish from ‘Case II’). Nucleotide sequences of PCR products shared 100% similarities with seven sequences deposited at GenBank (accession numbers: DQ085626.1, DQ085627.1, DQ085628.1, HM014349.1, JQ740764.1, JQ740765.1, and AB709903.1). They also shared 99% sequence homologies with another two sequences (AY939863.1 and KC841411.1), indicating that these goldfish were infected by CyHV-2.
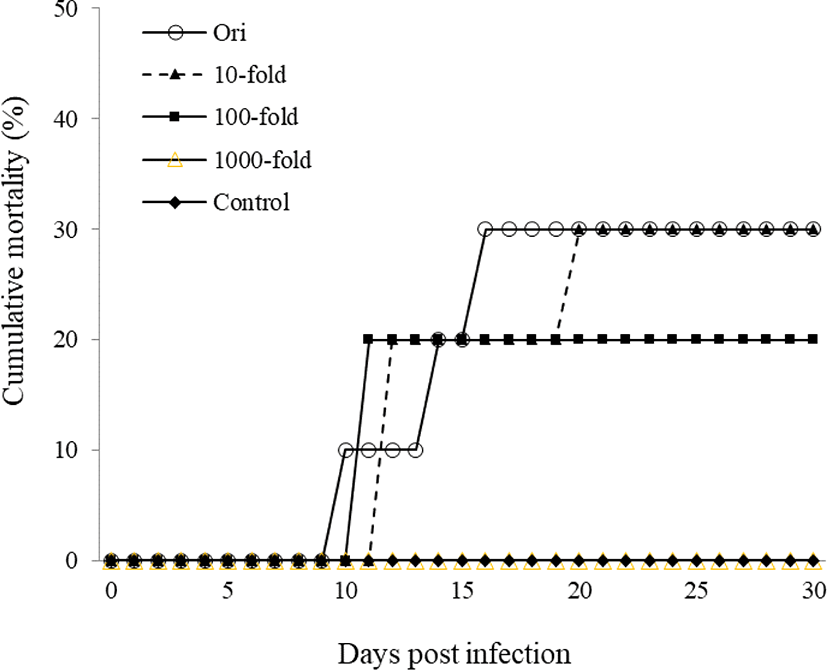
For experimental infections, a total of 60 healthy goldfish were i.p. injected with kidney and spleen filtrate (100 μL/fish) at the original concentration and at 10-, 100-, and 1,000- fold dilutions. Original concentration group began to die from 10 days post infection and cumulative mortality reached 30% for 30 days. Mortalities in the 10-, 100-, and 1,000-fold diluted groups were 30%, 20%, and 0%, respectively. However, the control group did not show any mortality (Fig. 2). CyHV-2 infection was confirmed by amplification of CyHV-2 polymerase gene product from kidney of dead goldfish.
Naturally infected fish in ‘Case I’ showed extensive necrosis in the splenic pulp and hematopoietic tissue of the kidney. Some enlarged nucleus with peripherally displaced chromatin, which is characteristic of CyHV-2 infection, was shown in the spleen and kidney (Fig. 3A and 3B). Only in ‘Case I’, bacterial colonies were existed in the macrophage-like cells in the spleen and kidney (Fig. 2A). Fusion of secondary gill lamella by abnormal hyperplasia of gill epithelial cells was found but there was no necrotic change. Liver parenchymal cells were not damaged but had many cellular debris in expanded central veins. Cellular debris might be necrotised cells entering the central vein from other organs. In the intestine, the mucus epithelium was sloughed to the lumen with severe necrosis of epithelial cells. Natural infection in ‘Case II’ showed severe necrosis in the spleen and hematopoietic tissue of the kidney, including nuclei with karyopyknosis and karyorrhexis with some peripherally displaced chromatin in the nucleus (Fig. 3C). There was no specific change in other tissues. Histopathological change of dead goldfish from experimental infection exhibited extensive necrosis in the kidney and spleen (Fig. 3D). However, other tissues such as liver, heart, and intestine were normal. In the gills, the fusion of secondary gill lamella showed abnormal hyperplasia of gill epithelial cells, but at similar level to those in the non-infected control.
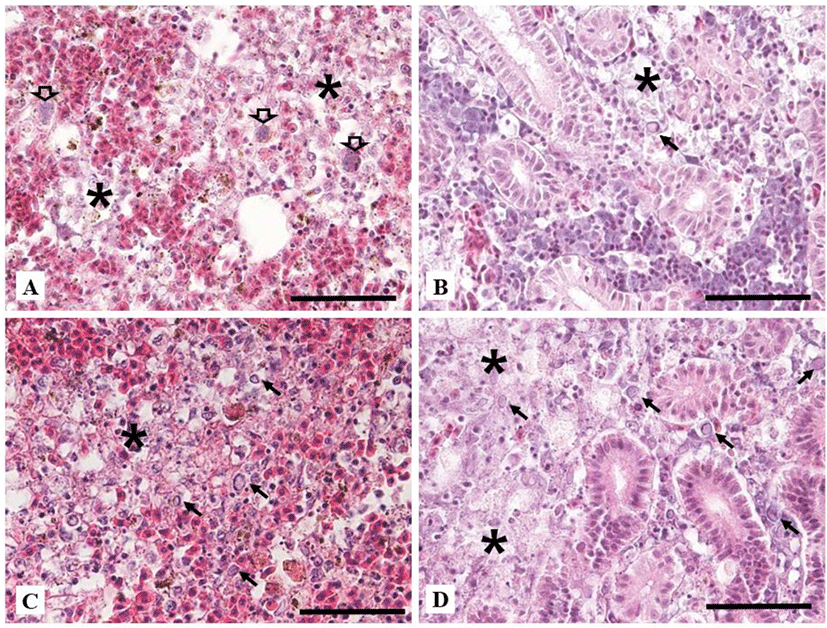
In other reports, necrosis by CyHV-2 infection has been shown in hematopoietic tissues of kidney and spleen with enlarged nucleus and peripherally displaced chromatin (Goodwin et al., 2006; Jung & Miyazaki, 1995; Stephens et al., 2004). Histopathological changes of ‘Case II’ and ‘experimentally infected fish’ were having similarly severe necrosis of hematopoietic tissue and splenic pulp. Peripherally displaced chromatin was also observed as previous reports. Histopathology of ‘Case I’ also exhibited characteristic histopathological changes of CyHV-2. However, it had additional alterations such as necrotic cells in blood vessels and sloughed intestinal epithelial layer into the lumen. These might be due to co-infection by Aeromonas sp.
Most ornamental fish including goldfish in South Korea are imported from the countries such as China, Singapore, and Japan. Generally, they are distributed to various places through online or local wholesale markets. Their disease status is mostly unknown. Goldfish died at aquarium in April 2014 in this study were bought from a local wholesaler collecting goldfish from various sources. Song et al. (2008) detected CyHV-2 by PCR from December 2016 to November 2017 then showed 33.3% of pearlscale goldfish C. auratus imported from Singapore were positive for CyHV-2. Surprisingly, similar percentage, 27.8%, of domestic goldfish were also CyHV-2 positive means the virus has already been widespread throughout Korea at the year 2016 and 2017. Not much attention has been paid to disease diagnosis or disease etiology of ornamental fish because of lower economic value. More attention is needed for ornamental fish to prevent foreign pathogens become indigenous.
