Introduction
Ornamental fish exhibit a variety of attractive features, such as body color, body shape, and suitability in aquariums (Khomdram, 2018). Various types of ornamental fish are traded on a global scale with approximately 5,000–6,500 freshwater species and 1,600 marine species (Moorhead & Zeng, 2010). According to Moorhead & Zeng (2010), the ornamental fishing industry has reached 15 billion US dollars (USD).
Growing global demand for ornamental fish at rising prices has not been followed by sustainable conservation and farming efforts because of growing pressures (Nunez et al., 2019), especially for wild-caught fishes, due to over-fishing, environmental degradation (Lotze et al., 2006), or climate change (Beaugrand & Kirby, 2018). Among the aforementioned problems, freshwater ecosystems face more severe problems than seawater ecosystems (IPBES, 2019). However, conservation efforts have been relatively less extensive than in seawater ecosystems (Strayer & Dudgeon, 2010).
The Celebes rainbow (Marosantherina ladigesi) is a type of Indonesian ornamental fish that has been exported since 1976. One of the major importing countries is Germany (Hadiaty, 2007). Exports of rainbow fish depend solely on wild catches, and while these fish may be found in significant quantities in rivers some time ago, unlimited fishing even involves poison (Hadiaty, 2007) and changes in the aquatic environment due to settlements and agricultural activities around the rivers (Gebrekiros, 2016) make the fish increasingly difficult to find and indicate that they have almost dissapear.
Research on Celebes rainbow is limited, especially regarding its cultivation. There have been no specific research reports studying the use of feed at the domestication stage; therefore, this study focused on rainbow fish culture by trying several types of feed by adopting the feeding habits of fish in nature. According to Bilio (2007), feeding habits are important information in the context of limited aquaculture activities. Feeding habits for each species of fish vary, but feeds containing fishmeal and live food are usually more readily accepted by domesticated fish (Li et al., 2019). On the other hand, differences in type, nutrition, and many other differences, will affect feed utilization and ultimately fish growth (Hien et al., 2016). The feed must be available in sufficient quantities, ready to eat and digest effectively and contain nutrients that support fish growth and health. Therefore, it is important to gradually introduce new types of feed to allow the fish to adjust to the type of feed during the rearing period which should be cheap, readily available, and sustainable (Dietrich et al., 2021). However, in Celebes rainbow, no information on suitable foods at the domestication stage, which is why research on four types of feed with different characteristics was tested; live Tubifex sp, dry Tubifex sp, artificial crumble pellets, and natural feed Spirulina platensis. These four experimental feeds have almost the same nutritional content, in particular protein, but have different physical and biological characteristics
The main objective of this pilot study was to investigate the potential of several types of optimum feed to improve the absolute growth rate (AGR), specific growth rate (SGR), survival rate (SR), feed conversion ratio (FCR), feed efficiency (FE), hematology, immunity and resistance to pathogen of Celebes rainbow.
Materials and Methods
The experimental animals were male Celebes rainbow obtained from the Bantimurung river, South Sulawesi, Indonesia. The fish were kept in the laboratory for 14 days for acclimatization to study conditions, size selection, and determination of fish health status. Acclimatization is also necessary for changing the habits of fish that are lotic in the wild. A total of 360 male fish with an average weight of 1.32 ± 0.21 g/individual and standard length of 4.0 ± 0.55 mm were randomly stocked into 12 aquariums with a volume of 54 liters (30 fish/aquarium). During feed treatment, water parameters were maintained within the same range as in the wild: water temperature 27°C–29°C, dissolved oxygen 6.0–6.4 ppm, pH 7.0–7.3, alkalinity 115–141 ppm, and hardness 109–125 ppm. A change of water was done at 20% daily in addition to siphoning which was done at any time to remove accumulated residual feed or fish feces.
The four types of feed tested consisted of live Tubifex sp worms, dry Tubifex sp worms, flour S. platensis meal, and artificial crumb feed. Live Tubifex sp worm was prepared under optimal culture condition provided by the aquaculturist surround, while S.platensis, dry Tubifex sp, and crumble feed are commercial products. Each test feed was given randomly to an aquarium filled with fish with three repetitions, and the fish were hand-fed to satiation twice daily for six weeks. The nutritional content of the test feeds is presented in Table 1.
The design used in this study was a completely randomized design with four types of feed treatments, consisting of live Tubifex sp worms, dry Tubifex sp worms, S. platensis meal, and artificial crumble pellets, each triplicate.
The research parameters consisted of the AGR, SGR, SR, FCR, FE, total leukocytes, phagocytic activity, hematocrit (Ht), hemoglobin, total erythrocytes, intestinal and liver histology, and fish immune system assay. Growth performance was observed by weighing 15 fish/aquarium at the beginning and the end of the study. The fish’s AGR, SR and FE were calculated based on Busti et al. (2020) and Farhad et al. (2023). Fish hematology was observed by randomly harvesting three fish per study unit at the end of the study (on the 42nd day) and anesthetizing them using MS-222 (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 50 mg/L (Priborsky & Velisek, 2018). The hematological parameters total leukocytes, total erythrocytes, and the differential leukocyte were carried out based on Blaxhall & Daisley (1973), while the phagocytic activity was based on Anderson & Siwicki (1993) and Ht based on Alexpandi et al. (2020).
To observe the development of the fish intestines and the storage of energy reserves in the fish liver, at the end of the study, histological preparations were made from three fish from each type of feed treatment. To make histological preparations, the fish were anesthetized with MS 222 (50.0 mg/L), then the fish was carefully dissected through the ventral cavity. The fish livers and intestines were removed and fixed in 10% formaldehyde (Ogueji et al., 2020) and processed using histological methods. Tissues embedded in paraffin were sliced 5 μm thick and stained with hematoxylin and eosin. The slides were observed using a light microscope to see changes in the intestinal and liver tissues after being treated with several types of feed. Intestine and liver histology results were observed descriptively. Intestine histology of fish is described and scored based on the growth of the fish intestinal villi, presence of goblet cells and lamina propria, as well as liver histology based on differences in the cohesiveness of the uterine cells containing glycogen as energy reserves.
The challenge test was conducted at the end of the study by rearing the fish for seven days in a 5-liter container, each duplicate. The infection was done by spreading Aeromonas hydrophila at a density of 107 CFU/mL into a receptacle containing 2 liters of aerated water. After the bacteria are homogeneous, five fish were put into the container. After 24 hours, the fish were transferred to a 5-liter container of water and reared for seven days. The accumulation SR was observed daily until the 7th-day post-challenge test.
The data for AGR, SGR, SR, FCR, FE, hematology, and SR post-challenge test were statistically analyzed using SPSS version 22 (IBM, Armonk, NY, USA) with one-way analysis of variance test followed by with Tukey’s multiple range test to determine the effect of treatments. The histology of the fish intestines and liver were analyzed descriptively.
Results
The survival of the Celebes rainbow fish during the study period (Table 2) ranged from 64.45%–83.33%. The highest survival percentage of the tested fish was found in the fish-fed live tubifex, followed by fish-fed artificial feed, S. platensis meal, and the lowest in dry Tubifex. In general, it demonstrated that the test feed could be utilized by the fish. This is closely related to the omnivorous eating habits of rainbow fish.
Each value represents mean ± SE (n = 3). Differences between means were tested with Duncan’s multiple range test.
Fish growth measured by AGR and SGR (Table 2) showed an increase during the study. The different feeds had a significant effect on fish growth (p < 0.05). Tukey’s follow-up test showed that the highest AGR, SGR, and final weight of fish were found in the fish-fed live Tubifex sp worms, then followed by the artificial feed treatment, S. platensis meal, and lastly dry Tubifex sp worms (p < 0.05).
The FCR and FE of rainbow fish given each of the four types of feed treatments are presented in Table 2. Tests with the four different types of feed showed a significant effect on both the FCR and FE (p < 0.05). Among the types of feed tested, the application of live Tubifex sp demonstrated the lowest FCR in rainbow fish compared to fish in other treatments, whereas the highest FCR was observed in dry Tubifex sp feed. Likewise, the FE in fish treated with live Tubifex sp feed was more efficient than the other treatments, and the lowest FE was in the dry Tubifex feed (p < 0.05).
The hematology of the test fish in this study all exhibited the effects of the four feed treatments (p < 0.05; Table 3). The four types of different feed showed different increases in the fish hematology, but the live Tubifex sp feed had the highest and most significant increase in hematology for all the hematological parameters compared to the dry Tubifex sp and S. platensis feeds.
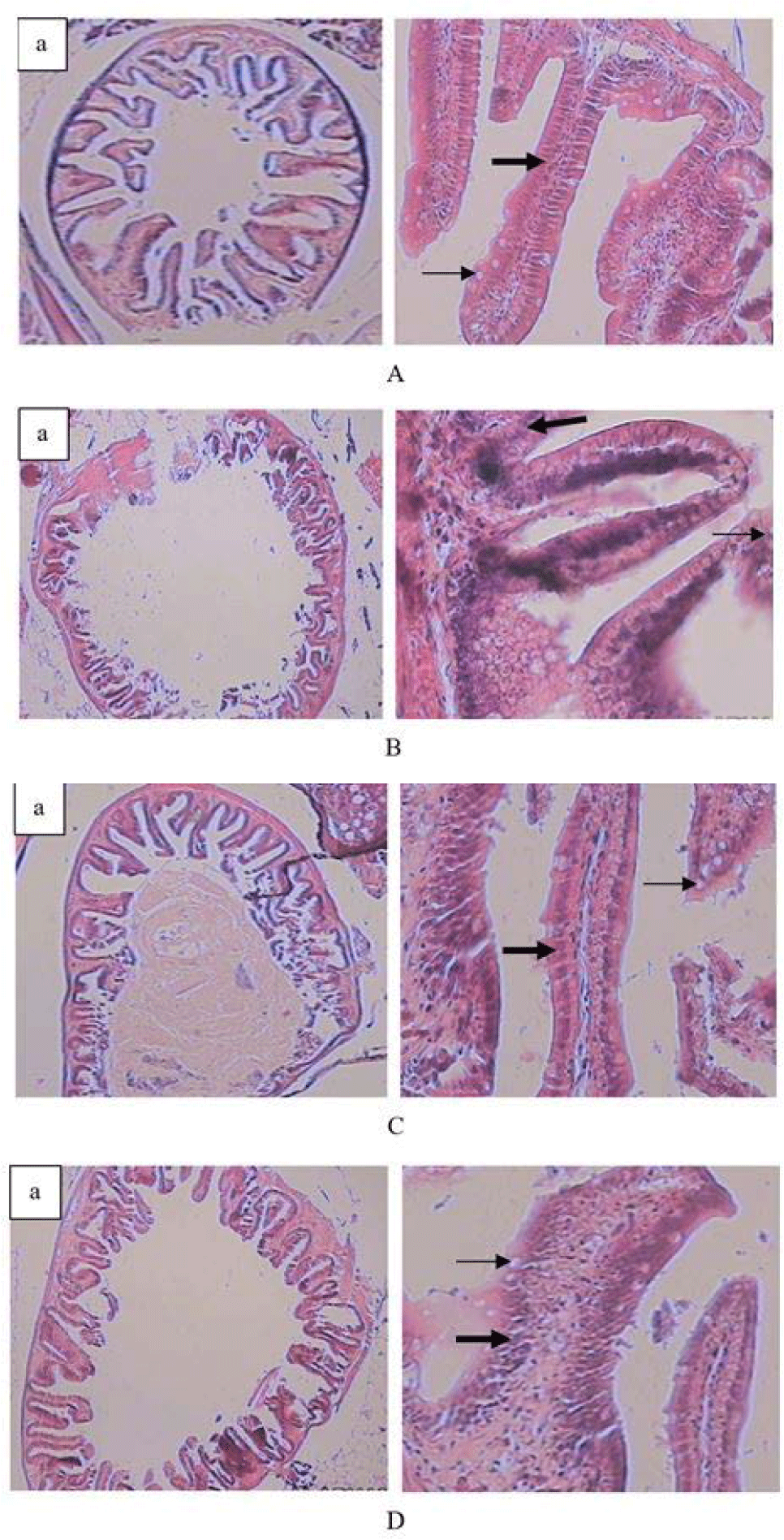
Descriptively, the fish’s intestinal and liver histology exhibited differences in transverse intestinal slices, intestinal villi slices, and liver slices between the test fish based on feed treatment. In the fish fed live Tubifex, the intestinal villi were well-developed and were denser, allowing them to break down the feed mechanically, the number of lamina propria, goblet cells, and mononuclear immune cell infiltration were also higher, especially in fish that were fed live Tubifex sp and commercial feed, whereas the fish fed dry Tubifex sp and S. platensis had fewer lamina propria and fewer goblet cells (Table 4 and Fig. 1).
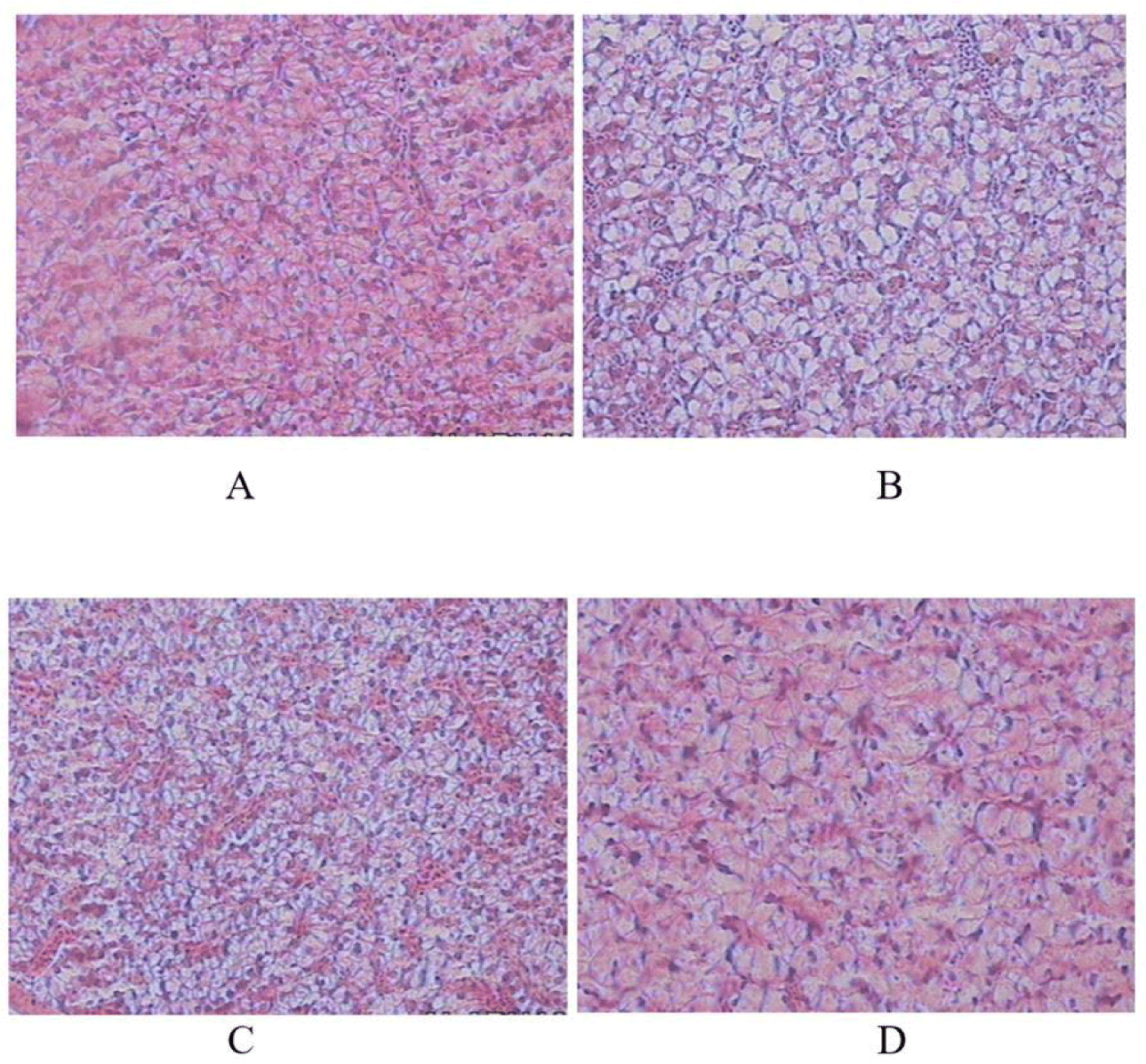
The histology of the fish’s liver also exhibited differences in terms of cell cohesiveness, glycogen content, which is an energy reserve, and fat content. The livers of the fish-fed live Tubifex sp were better than those of the other 3 treatments (Fig. 2).
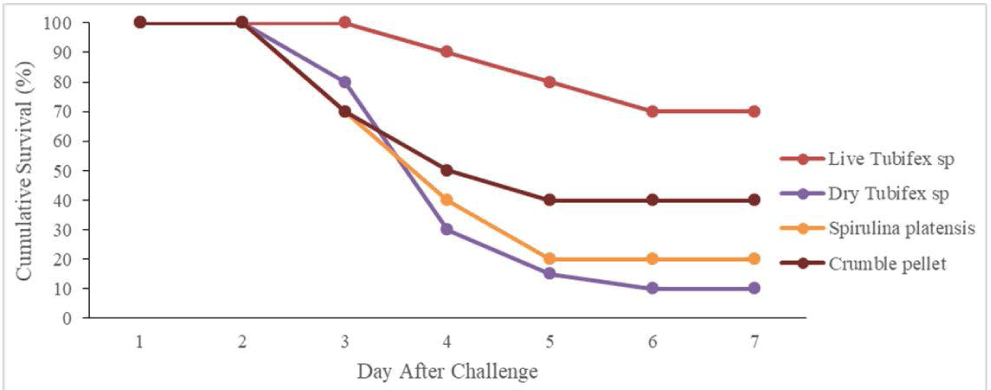
A challenge test was conducted to determine the fish’s resistance to Aeromonas after six weeks of rearing using different types of feed. The SR accumulation post-challenge test until the 7th-day post-challenge test can be seen in Fig. 3. The fish’s SR began to decrease on the 3rd day after the challenge test indicated by fish mortality in the treatment using dry Tubifex sp, S. platensis, and crumble pellets test feeds. SR accumulation was lower after high fish mortality in all treatments from day 4 to 7 post-challenge test. The statistical analysis of the fish’s SR on the 7th-day post-challenge test showed different fish resistance to infection based on the type of test feed (p < 0.05). Tukey’s follow-up tests showed that fish-fed live Tubifex sp had the highest resistance to disease (p < 0.05) while the lowest was found in fish-fed dry Tubifex sp and S. platensis.
Discussion
The study of maintaining Celebes rainbow with the input of various types of feed has been conducted. This study showed that Celebes rainbow fish could utilize all types of feed tested, although based on statistical tests live Tubifex sp was better than the other types of feed tested (p < 0.05). The fish fed live Tubifex sp worm performed better AGR, SGR, SR, FE, hematology, immunity, and lower FCR compared to pelleted feed, S. platensis, and dry Tubifex sp (Tables 2 and 3). These results are in line with the use of Tubifex sp worms in guppy fish (Poecilia reticulata; Perera & Bhujel, 2022) and Clown loach fish, Cromobotia macracantus (Putra et al., 2019), demonstrating the best growth and SR. A similar study about several types of feed on Notopterus chitala seeds for 60 days showed that Tubifex sp increased growth and digestive enzyme activity, and improved the SR (Sontakke et al., 2019). Likewise, the use of Tubifex sp worm feed for eight weeks (Arslan et al., 2009) resulted in the highest growth rate among live feeds, commercial feeds, and formulated feeds on juvenile South American catfish, surubim (Pseudoplatystoma fasciatum). The use of Tubifex sp worms also increased the SGR and resulted in the highest SR of Chitala chitala after rearing for 28 days (Sarkar et al., 2006).
Hematological observations in this study showed results in the optimal range for the total leukocytes, phagocytic activity, total erythrocytes, Ht, and hemoglobin (Table 3); all these play an important role in the fish’s immunity and nutritional metabolism. Hematological parameters are important determinants for assessing fish feed quality and fish health status (Tabassum et al., 2021). This study is also similar to one about the domestication of the Mandarin fish (Siniperca sp) which affects growth performance by influencing appetite regulation, digestive enzyme activity, and immune response (Li et al., 2023), gut microbiota, and metabolic analysis (Li et al., 2023).
Although fish fed with Tubifex had higher hematology than other treatments, in general, fish in all treatments showed optimal hematology. This indicates that the physiological condition of fish with feed treatment during the study was normal. Hematology parameters; total leukocytes and phagocytosis activity play a role in fish immunity. Hemoglobin plays a role in metabolism, red blood cells (RBCs) play a role in oxygen transportation to tissues, and Ht determines the volume of RBCs (Taherpour et al., 2023).
The high hematological parameters in this study, especially in fish-fed Tubifex sp, are in line with the study by Abarike et al. (2022) who used several natural ingredients in tilapia. Total leukocyte and phagocytic activity play an important role directly in the fish’s non-specific immune response (Abarike et al., 2022; Chen et al., 2021). This study also showed that the application of live Tubifex sp increased the Celebes rainbow’s resistance to pathogenic diseases, especially post-challenge test with A. hydrophila (Fig. 3). The challenge test conducted after fish rearing showed that up to day 7, Tubifex sp-fed fish had the highest resistance to A. hydrophila infection compared to the other treatments. The high resistance of fish treated with Tubifex sp is in line with the high hematology of fish, namely total leukocytes and phagocytosis activity (Kim et al., 2021a, 2021b; Table 3) and also many mononuclear immune cells and goblet cells in the intestine (Table 4) which play a role in increasing the immune response. This is especially true in fish-fed S. platensis and dry Tubifex sp, where the activity of pagosity, total leukocytes, mononuclear immune cells, and goblet cells in the intestine is lower, making them more susceptible to attack by pathogenic bacteria.
Although the four types of feed tested had relatively the same nutritional content, there were differences in the fish’s AGR, SGR, SR, FCR, FE, immunity, hematology, and disease resistance. Some of the advantages of Tubifex sp compared to other feeds include the size of the Tubifex sp feed which is ideal for the size of the fish’s mouth and that live Tubifex sp will float in the water and will be chased by fish (Busti et al., 2020). Tubifex sp worms also do not have a skeleton so they are easily digested by the fish. In addition, the smell and color of live Tubifex sp worms stimulate fish to eat them (Budiardi et al., 2005). The nutritional components of Tubifex sp are rich in n-3 (C18: 3n-3 and C20: 5n-3), n-6 fatty acids (C18: 2n-6 and C20: 4n-6). Tubifex sp worms contain ten essential amino acids; arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, valine, and tryptophan.
The ability of fish to digest a feed depends on the feed’s physical and chemical characteristics, the type of feed, the nutritional content of the feed, the size and age of the fish, the type of digestive enzymes, the feeding frequency, the water temperature and chemical properties (Jannathulla et al., 2019). In addition, live Tubifex sp worms are more palatable to rainbow fish (Jannathulla et al., 2019; Yadav et al., 2020), directly affecting fish growth (Jingting et al., 2020). Live feed such as Tubifex sp contains all the nutrients required by fish such as essential proteins, lipids, carbohydrates, vitamins, minerals, amino acids, and fatty acids (New, 1999), therefore the type of feed applied will play a significant role in feed consumption and growth, as well as its effect on the reproductive aspects of fish. Several studies using live Tubifex sp feed have shown an increase in fish growth performance, including the ornamental fish C.chitala (Sarkar et al., 2006), Betta splendens (Mandal et al., 2010) and Guppy fish (Busti et al., 2020). This study also showed that the application of live Tubifex sp increased the Celebes rainbow’s resistance to pathogenic diseases, especially A. hydrophila.
Fish fed with dried Tubifex sp, S. platensis, and artificial feed showed lower performance in all test parameters compared to fish fed with live Tubifex sp. Physically, Spirulina meal is very small in size so it has a large percentage of not being eaten by the fish. Similarly, the artificial feed will be destroyed, thus both types of feed will tend to spread and ultimately not be eaten by fish and can even cause damage to the maintenance water. While the dry Tubifex sp test feed had low palatability compared to the live Tubifex sp. The physical and biological deficiencies of the test feed caused low feed consumption. It is different for fish fed with live Tubifex sp. Although in this study the application of the crumble feed formula improved growth performance, immunity, and resistance not as good as with live Tubifex sp but better than other test feeds, thus the application of crumble feed can be used to replace live Tubifex sp. According to Mandal et al. (2010), the disadvantages of live food such as Tubifex sp are not available at all times which makes it impractical and may contain diseases that can be transmitted to the fish that eat it.
Moreover, the lower growth performance of fish fed with dry Tubifex sp, S. platensis, and crumble feed was due to the high carbohydrate content. According to Rahman et al. (2023), feed containing high carbohydrates and fiber interfere with enzymes from accessing their substrates or directly interacting with enzymes decelerating digestive processes, thereby impairing the digestibility of feed ingredients. Information on the nutrient digestibility of feed ingredients is indispensable for improving the accuracy of diets for fish species in terms of formulating cost-effective feeds.
The intestinal histology (Fig. 1) of fish fed a diet of live blood worms showed denser, well-developed intestinal villi, allowing them to break down the feed mechanically. The development of these intestinal villi indicates the quality of the feeds tested during rearing (Goda et al., 2020). The villi cell development was almost the same as the intestinal villi in fish-fed artificial feed.
Intestinal villi increase the absorptive surface area while enterocytes are absorptive cells, acting as the main structural component of the villous epithelium, and regulating the passage of nutrient molecules in aquatic animals (Tabassum et al., 2021). Goblet cells are a type of intestinal epithelial cell that secrete mucin, intestinal trefoil factor, and resistin-like molecules which coat the surface of the intestinal tract (Chen et al., 2021). Under certain conditions, mucus secretion will increase, including those related to the immune response that will play a role in protecting fish from disease (Tabassum et al., 2021). Therefore, histological analysis of the intestinal tract of fish is important to evaluate changes at the tissue level of fish during feed testing (Yadav et al., 2020).
The histology of the liver (Fig. 2) showed differences in terms of cohesiveness, where the livers of fish-fed live Tubifex sp had a dense and compact liver structure. It can also be seen that the dominantly red color indicates the amount of glycogen which indicates that energy reserves are higher and the fat content is low; on the other hand, in the treatment of dry Tubifex sp worms, the livers are dominated by fat and are low in glycogen. This supports the previous statement that live Tubifex sp feed has better digestibility.
Accumulation of fat in the liver is the most determining parameter for larval growth and liver metabolic performance of a type of feed. A normal liver with rich hepatocyte glycogen, sinusoids, and central veins suggests that the diet does not cause gastric upset. The opposite can result in poor feed absorption and digestion in fish. However, there were no signs of liver damage in any of the fish, indicating that the feeds tested had a positive effect as a source of energy for the fish (Chen et al., 2021).
