Introduction
Ethiopia is endowed with huge amount of inland freshwater systems that belongs to 12 major river basins with mean annual flow of 122 billion metric cube and more than 36 lakes with surface area of about 7,500 km2 (FAO, 2017; Gebremedhin et al., 2018; Tesfaye et al., 2020). These water bodies sustain diverse economically and ecologically important fish species of which Nile tilapia, O. niloticus is by far the most commercially important fish of the country in general and traditional fishery of Lake Tana in particular (Assefa & Getahun, 2015; Engdaw et al., 2013; Tefera et al., 2019; Tesfaye et al., 2020). The species is distributed in many tropical and subtropical African aquatic systems and also native to Lakes of Tanganyika, Albert, Edward, Kivu, Chad and throughout Nile River basin (Njiru et al., 2004; Shipton et al., 2008; Yirga & Brook, 2018). O. niloticus is distributed in all inland waters of Ethiopia including lakes, rivers reservoirs and swamps contributing 60% of the total landings of fish (Fetahi et al., 2017; Tesfaye et al., 2020).
Suitability to various culture techniques and its tolerance to numerous environmental and physico-chemical water quality parameters such as frequent fluctuation in pH and salinity, low dissolved oxygen, high concentrations of chemical nutrients, ability to reproduce in captivity and potential to accept natural and formulated feeds make the species most preferred and best cultured fish around the globe (Assefa & Getahun, 2015; Engdaw et al., 2013; Gule & Geremew, 2022; Suloma et al., 2017; Tesfahun & Temesgen, 2018; Tesfaye et al., 2020; Wagaw et al., 2022). Subsequently, commercial and ecological importance of Nile tilapia is undeniable especially in many top tilapia farming Asian and African countries where the demand for inexpensive protein diet is alarming exponentially (Ribeiro et al., 2018). Hence, prolific measures such as feeding and management with especial emphasis on improved nutrition are essential (Mabroke et al., 2013; Sousa et al., 2013).
Knowledge on morphometric (length-length and length-weight) relations are critical aspects in determinations of growth rates, average weight of a given length group, and population age structure of the species in different aquatic systems (Bolarinwa & Popoola, 2013; Engdaw, 2014; James et al., 2000; Zannat et al., 2020). Furthermore, morphometric relations are vital in verifying relative well-being of fish in stock management and environmental quality of the habitat types (Ondhoro et al., 2020). Therefore, comprehensive understanding on morphometric relations enables improvement of optimal strategies for effective conservation and stock management against fishing pressure and other ecosystems dynamics (Ondhoro et al., 2020).
Apparently, studies on the food and feeding habits of fish species has become a focus of research due to strong global demand for the development of successful capture and culture fisheries management program (Gule & Geremew, 2022; Tesfahun & Temesgen, 2018). It is also essential for pond production management, formulating dietary needs of the species used in intensive and extensive culture and also in determining whether the population age structure in the fishery is in proper relation to the food resources available to it (Engdaw, 2014; Wagaw et al., 2022). Recent studies elucidated that, knowledge on feeding habits of fish enables to identify trophic interactions, composition, structure and stability of aquatic food webs (Fetahi et al., 2017; Tesfaye et al., 2020).
In nature fishes feed on a wide variety of food items such as phytoplankton, zooplankton, benthic and non-benthic invertebrates, detritus, aquatic macrophytes and other fishes (Dadebo et al., 2014; Engdaw, 2014; Hussian et al., 2019; Ondhoro et al., 2020; Zannat et al., 2020). However, feeding habits of fish might vary due to species, age, size, maturity stage, habitat (littoral or profundal), water type and water level of the environment (Dadebo et al., 2014; Persson & Crowder, 1998). Moreover, seasonal variations and lake type have a significant influence on the feeding habits of the fish (Engdaw et al., 2013; Wagaw et al., 2022).
Despite numerous studies have been conducted and a great deal of information is available on the food and feeding habits of O. niloticus from various Ethiopian inland water bodies (Assefa & Getahun, 2015; Fetahi et al., 2017; Getachew, 1993; Negassa & Prabu, 2008; Tefera et al., 2019; Teferi et al., 2000; Tesfahun & Temesgen, 2018; Tesfaye et al., 2020; Tudorancea et al., 1988; Yirga & Brook, 2018), a consistent pattern has not yet emerged. According to the above mentioned studies, O. niloticus in Ethiopian inland water bodies was predominantly considered as omnivorous, mainly consuming phytoplankton, zooplankton, detritus and other aquatic macrophytes. Furthermore, almost all the above studies are conducted in the rift valley lakes and their tributaries due to location and chance for comparison (Fetahi et al., 2017; Tesfaye et al., 2020; Wagaw et al., 2022). Nevertheless, there is paucity of information on the morphometric relations and feeding habits of O. niloticus from Ethiopian highland Lakes such as Lake Tana. Siltation, improper waste discharge, habitat destruction, recent invasive alien water hyacinth infestation, recruitment of the young, rapid population growth causing over exploitation, excess nutrient enrichment resulting eutrophication and lack of integrated fisheries management combined with hydrological alterations are currently threatening Lake Tana and its biodiversity to continue as a resilient ecosystem (Dersseh et al., 2020; Endgaw, 2020; Gebremedhin et al., 2018; Goshu & Aynalem, 2017). Additionally, area specific information is essential for maintaining good ecological integrity and sustainable use of resources. Therefore, the purpose of this study was to update morphometric relations, diet compositions and ontogenetic dietary shift of O. niloticus in Lake Tana, gulf of Gorgora.
Materials and Methods
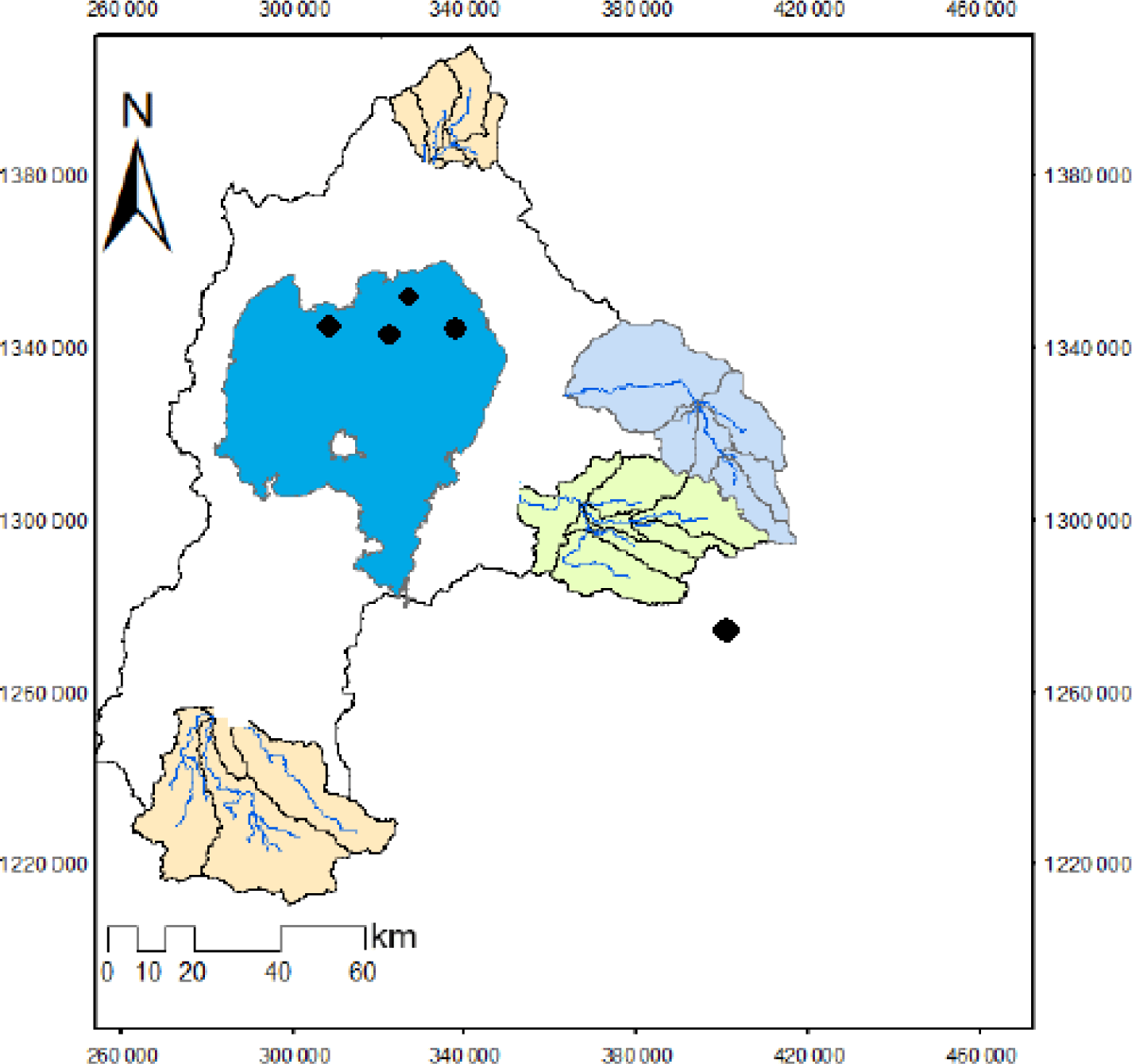
Lake Tana is the largest lake of Ethiopia and Africa’s top 32 with global rank of 250 for conservation priority (Goshu & Aynalem, 2017; Teshome et al., 2021). It is located in the North West part between 12°10’N and 37°23’E with an altitude of 1,800 meters above sea level (Fig. 1). The total surface area of the lake is estimated about 3,050 km2 with maximum and mean depths of 14 m and 8 m respectively (Dersseh et al., 2020; Engdaw, 2014). The water from the lake is used for hydropower generation, fishery production, irrigation, water supply and waste disposing site. Lake Tana is the main source of Blue Nile River with oligotrophic character (Endgaw, 2020; Gebremedhin et al., 2018). Due to its religious, ecological and socio-economic benefits, in 2015, the lake was registered by UNESCO as world’s heritage of biodiversity and biosphere reserve (Mequanent & Mingist, 2019; Teshome et al., 2021). Apart from the endemic fauna and flora, presence of ancient monasteries in the highlands and historical sites make the lake most precious natural resource serving as heritage (Tefera et al., 2019). However, recent severe anthropogenic activities including rapid population growth seeking for food and water security, habitat destruction and huge invasive alien water hyacinth macrophyte infestation are causing a devastating impact on Lake Tana’s biodiversity and ecosystem integrity. Out of the total 28 fish species that inhabit Lake Tana, 21 of them are endemic to Ethiopia including the IUCN registered Labeobarbus species flock. Currently, there is huge stock decline in the fisheries of Lake Tana due to high fishing effort using commercial monofilament gillnet and also broad damage of spawning stock through poisoning; using crushed seeds of birbira (Milletia ferruginea) (de Graaf et al., 2004; Engdaw, 2014; Teshome et al., 2021).
A total of 309 fish samples were collected from various fish landing sites, captured using gillnets of different stretched mesh size (6 to 8 cm) from February to April, 2020. In order to obtain juveniles, beach seine (10 m long and 3 m wide with mesh size of 3 mm) was used in the shallower part of the lake. Fish samples were identified using identification keys developed by (Golubtsov et al., 2002). Morphometric parameters such as, total length (TL), standard length (SL) and total weight (TW) of all specimens were measured using measuring board and sensitive digital balance (Model CY510, Citizen, Tokyo, Japan) to the nearest value of 0.01 cm and 0.01 g respectively.
In order to determine length-length and length-weight relationships of O. niloticus, the least squared regression analysis of Bagenal & Tesch (1978) was adopted.
Where, TL = total length, SL = standard length, ‘a’ and ‘b’ = are Y intercept and slope of the regression line or equation, respectively.
Determination of ‘a’ and ‘b’ values were performed using a non-linear regression of which the curves fitting were carried out by chi-square test (Jin et al., 2015).
Length – weight relations were also estimated by using the formula.
Where, TW = total weight, TL = total length ‘a’ and ‘b’ are Y intercept of the equation and slope of the regression line respectively.
Conversely, confirmation of the length-weight relationships was done using the log transformation of the above equation (Bolarinwa & Popoola, 2013).
The average condition factor index (K) of O. niloticus which reflects the physical and biological fluctuations by interactions related to feeding conditions in Lake Tana was determined using the Fulton’s formula.
Where, K = Fulton’s condition factor of fish, TW = total weight, TL= total length, the factor 100 is used to bring K close to unity (Froese, 2006).
After dissecting the fish on its ventral side, gut contents of all specimens were collected, labeled and preserved in sampling tubes using 4% formalin solution. Thus, all samples were transported to the laboratory for further analysis. In the laboratory, stomach content of each O. niloticus was transferred into petri-dish for visual and microscopic inspection. Identification of food items was done under a dissecting (LICAMS5) and Olympus compound microscope (magnification 10 to 40×). Food items were identified to the lowest possible taxa using various aquatic flora and fauna literatures (Fernando, 2002; Komarek & Anagnostidis, 1989; Pennak, 1978; Whitford & Schumacher, 1973). The relative importance of each food item was estimated using frequency of occurrence and volumetric methods of Windell & Bowen (1978).
The number of stomach specimens containing one or more of a given food item was expressed as a percentage of all non-empty guts examined. Proportions of the population that feed on particular food item were estimated and the frequency of occurrence was computed (Bowen, 1983; Hyslop, 1980).
Where, Fi = frequency of occurrence of the i food item in the sample; ni = number of guts in which the i item is found; n = total number of guts with food in the sample.
Food items found in the stomach contents of sampled fish were sorted into different taxonomic categories. The water displaced by a group of items in each category was measured in a partially filled graduated cylinder and expressed as a percentage of the total volume of the stomach contents (Bowen, 1983).
To evaluate the relative abundance of different organisms and to assess the importance of each prey category in the fish diet, the index of preponderance (IoP) was calculated based on Natarajan & Jhingran (1961).
Where Fi is the frequency of occurrence of species i and Vi is the percentage composition by volume of species i; S is the number of prey types, to facilitate comparisons among species, IoPi was converted into percent IoPi (%IoPi).
Data collected from all parameters were summarized using SPSS Version 20. After the data was checked for normality using Shapiro-Wilk test, one way ANOVA was used to compare the importance of food items within the species and size class. Frequency occurrence and volumetric contributions were calculated as percent. In addition, the results obtained from morphometric relations and dietary shifts were interpreted using correlation and regression.
Results
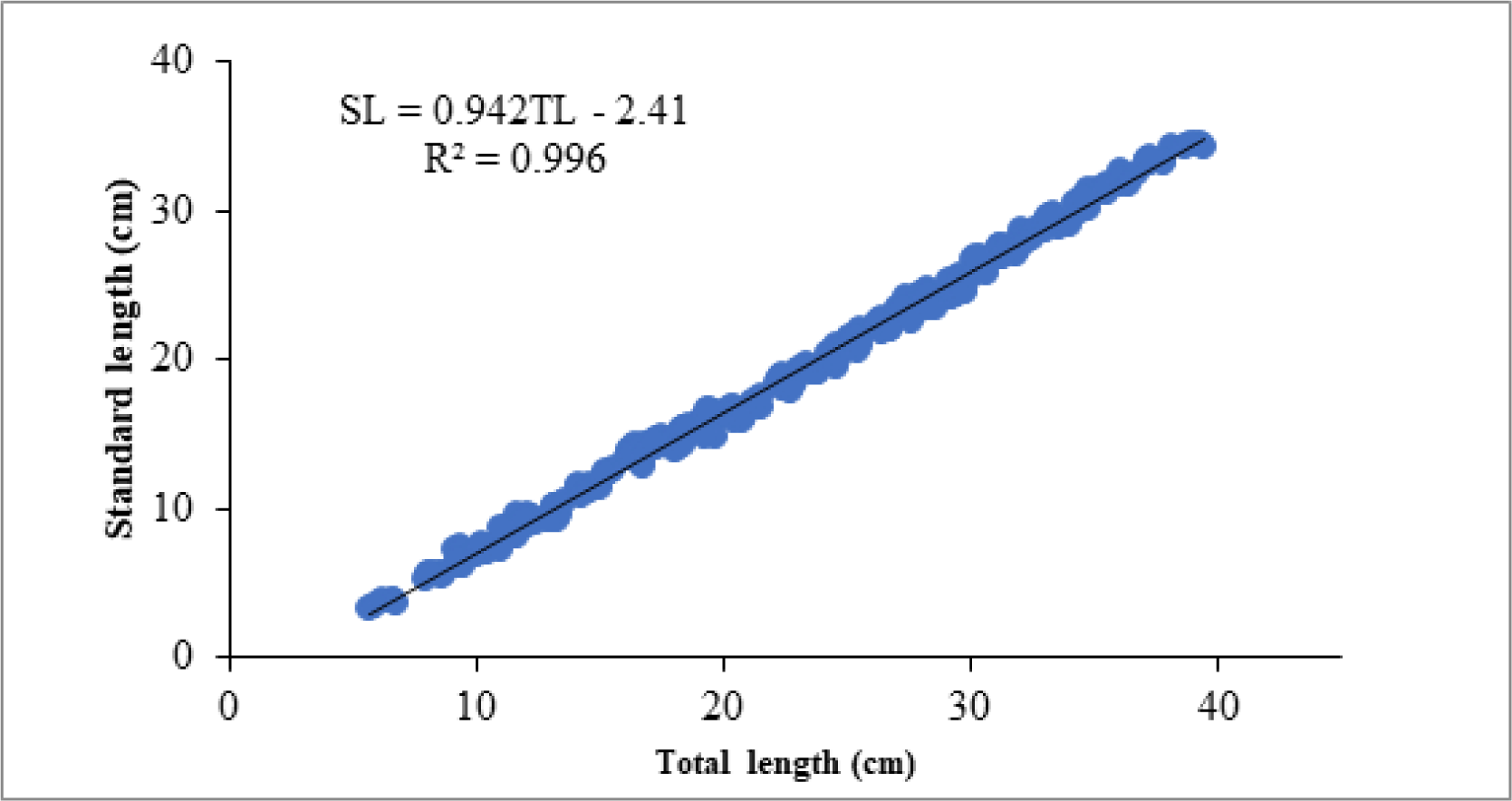
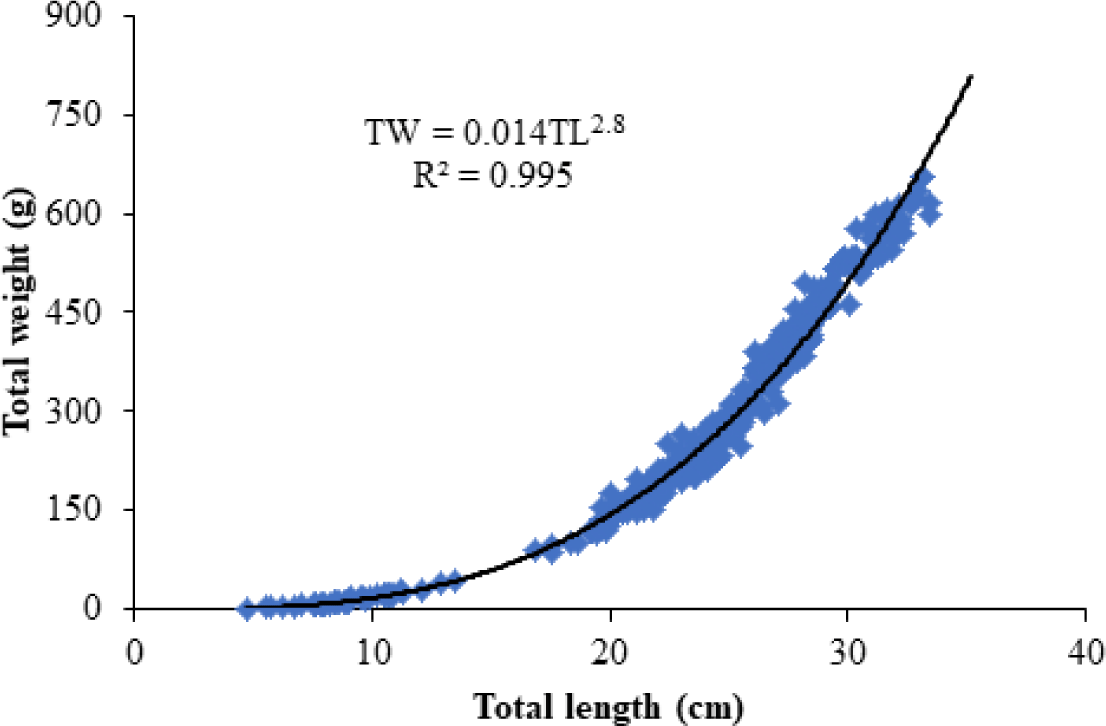
A total of 309 fish samples ranging from 5.7 to 38.5 cm in TL and 15.4 to 657.3 g in TW were used to determine the length-length and length weight relations of O. niloticus in Lake Tana, gulf of Gorgora. The relationship between TL and SL was linier with the equation of SL = 0.942TL – 2.41 and significant (p < 0.001) with R2 value of 0.996 (Fig. 2). The relation between TL and TW was curvilinear with the equation of TW = 0.014 TL2.8 and significant (p < 0.001, Fig. 3). The intercept “a” is 0.014 indicating the average condition factor index of the species and the slop “b” which is 2.8 indicates the growth pattern of O. niloticus.
Within the total 309 gut content specimens used to determine feeding habits of O. niloticus, 53 (17.3%) were vacant and the remaining 256 (82.7%) were non-empty with variety of food items. The most dominant food item in the diet of O. niloticus includes phytoplankton, detritus, macrophyte, zooplankton, insect larvae, nematodes and unidentified food items (Table 1). Out of the above mentioned food items, phytoplankton constituted largest bulk of the diet followed by detritus and zooplanktons giving an intermediate importance to the diet of the fish whereas the contributions of other food items such as macrophytes, insect larvae, nematodes and unidentified food items were low to the total diet of the fish (Fig. 4).
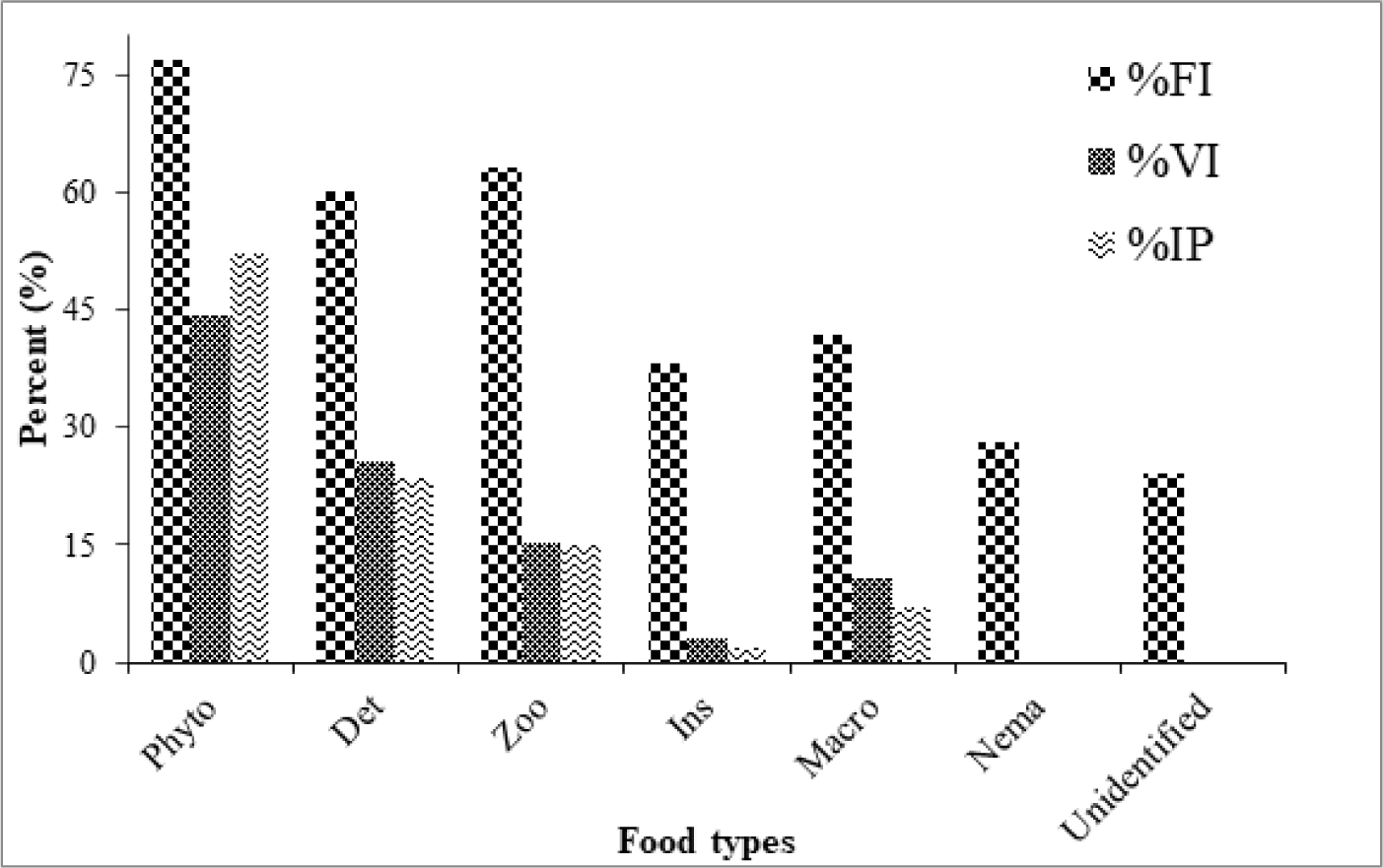
Gut content analysis revealed that, phytoplankton occurred in 77% of the guts examined and volumetrically accounted 44.3% of the total diet composition making them most important food items observed in the majority of guts examined (Table 2). Within the phytoplankton community, members of division Cyanophyta or blue green algae such as Microcystis, Planktolyngbya, Chroococcus and Lyngbya were dominant occurring in 69.5% of the specimens and constituting 20.3% of the total volume in the diet of the fish. Members of division Chlorophyta or green algae such as Scenedesmus, Botryococcus and Pediastrum had also a significant contribution in the diet of the fish by occurring in 48.4% of the gut and accounting 14.1% to the total volume. Other groups of the phytoplankton community including diatoms and euglenophyta also play an important role in the diet of the O. niloticus in Lake Tana (Fig. 4 and Table 2).
Detritus was the second most important and dominant food item in the diet of O. niloticusthat occurred in 60.2% of all gut contents scrutinized and constitute about 25.6% of the total volume of all food items (Table 2). Zooplankton which includes Rotifera, Copepods and Cladocerans occurred in 63.3% and volumetrically contributes 15.5% to the diet of the fish. Macrophytes were the other important food items that occur in 41.8% and constitute 10.9% of the total volume. Contributions of other food items such as insects, Nematodes and those unidentified food items occurred in 38.3%, 28.1%, and 24.2% of the entire gust examined and volumetrically constituted about 3.3%, 0.1%, and 0.3% to the total volume of the diet respectively.
The percentage IoP indicated that phytoplankton were the most preferred food items by O. niloticus in Lake Tana followed by detritus and zooplankton. In contrary, the preference of the fish to other food types such as insect, and nematodes were low (Table 1). Among phytoplanktons, members of the Cyanophyta and Chlorophyta specifically, the colonial forms such as Microcystis, Chroococcus; and single celled Botryococcus and Closterium were the most dominant food item (Table 2).
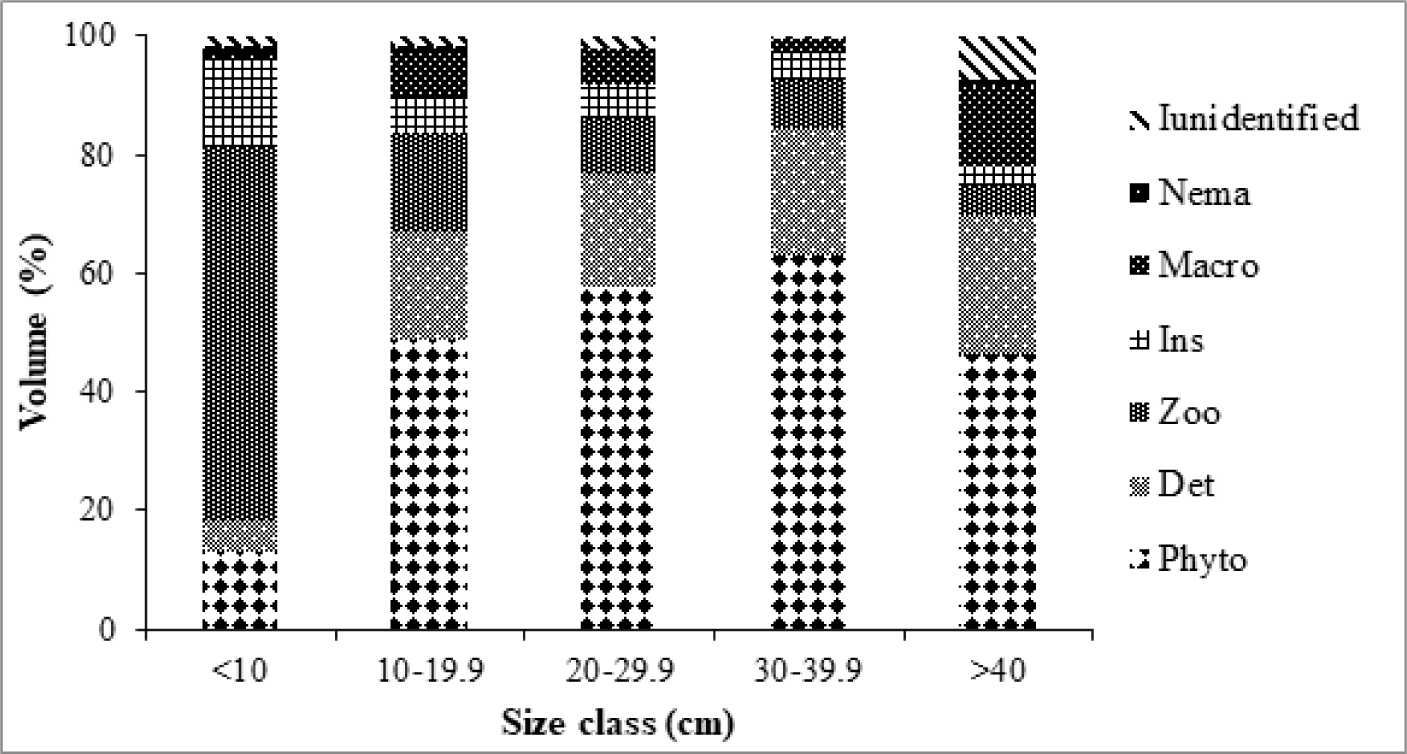
An attempt was made to determine presence of ontogenetic shift in the diet of O. niloticus. Significant change was observed in the diet of the fish as it increases in its size. All specimens were sorted into five size classes based on their TL. The first size class includes all samples of O. niloticus with TL < 10 cm, and zooplankton was the main diet of the category constituting about 63.2% of the total diet followed by insects (14.3%), phytoplankton (13%) and detritus (5.3%) with intermediate importance to the total diet of the size class. In the second group, samples between TL = 10–19.9 cm were included and a significant change was observed in the contributions of food items. In this size class, about half (48.9%) of the diet is accounted by phytoplanktons. Detritus, zooplankton and macrophytes contribute 18.3%, 16.5%, and 7.3% to the total diet of the group respectively while contributions of nematodes and unidentified food items remain insignificant in their contribution (Fig. 5). In the third and fourth size classes, i.e., TL = 20–29.9 cm and 30–39.9 cm respectively, phytoplanktons constitute the major food items in the diet of the fish by accounting about 57.6% and 63.1% to the total volume of the fish diet. Detritus, zooplankton and macrophytes remained in their intermediate importance. In the last size class of TL > 40 cm, phytoplankton remained as a major preferred food item while the consumptions of detritus and macrophytes showed a slight improvement to the total volume of the diet (Fig. 5).
Discussion
Morphometric data of O. niloticus in Lake Tana showed strong and highly significant relation. The linear relation between TL and SL indicated that either of the length measurements can be converted into the other or one parameter can be extrapolated from the other (Engdaw, 2014). In the curvilinear relations observed between TL and TW, the intercept ‘a’ indicated the average condition factor index of the species which is 0.014 revealing a value less than one (Kf < 1). This value suggested a state of wellbeing or suitability of the environment for the species tested. Kf > 1 indicates good growth condition while Kf < 1 designates the organism is in poor growth condition (Jisr et al., 2018). Therefore, O. niloticus in Lake Tana exhibited a poor growth condition. Reproductive cycles and breeding stages of the fish, availability of food, environmental parameters including habitat conditions seriously affect the growth rates of many fish species (Asadi et al., 2017). Similarly, seasonal variations in water temperature affect the metabolic activity of the fish leading to deprived growth condition. The average condition factor observed in the present study i.e., 0.014 is less than the finding of (Engdaw et al., 2013) from Koka reservoir which is 0.015. This might be due to the difference in availability of food or difference in time of sampling.
The slope of the regression line ‘b’ is an indicator of the specific growth pattern that the fish exhibits. The value of ‘b’ in the present study is 2.8, which is below 3 revealing allometric growth pattern of O. niloticus in Lake Tana. Despite the species dependent variation on growth rate of fish, food availability, gonad developmental stage, breeding condition and other environmental variables have significant impact on body shape and robustness of the species (Engdaw, 2014; James et al., 2000; Jisr et al., 2018). When the fish attains allometric growth pattern, it becomes slimmer and elongated or it becomes more slender as it becomes longer (Riedel et al., 2007). Apparently, due to the strong association between feeding habits of fish and their growth pattern, most carnivorous fishes attain positive isometric growth (b > 3.0) and most herbivorous fish attain allometric growth (Karachle et al., 2012).
Stomach content analysis of the present study disclosed that, about 17.3% of the guts scrutinized were vacant while 82.7% were non empty. The observed trivial number of vacant stomachs may be ascribed to the relatively short period of time between capture and dissection; and least possibility of food loss through digestion and regurgitation (Engdaw, 2014; Wagaw et al., 2022). It might also be due to the resisting ability of phytoplankton and detritus for digestion.
Variety of food types such as phytoplankton, zooplankton, detritus, macrophytes, insects, nematodes and unidentified food items were observed and reported in the gut contents of O. niloticus from many inland water bodies of Ethiopia (Assefa & Getahun, 2015; Negassa & Prabu, 2008; Tefera et al., 2019; Teferi et al., 2000; Tesfahun & Temesgen, 2018; Tesfaye et al., 2020; Wagaw et al., 2022). Result of this study also revealed ranges of food items and corroborate well with all the above-mentioned findings revealing herbivorous nature of the fish. In contrast, Gao et al. (2017) and Wagaw et al. (2022), supported the omnivorous feeding habits of O. niloticus, elucidating various food items in the gut contents of the fish including plants and foods of animal origins such as fish scale and invertebrates.
In the diet of adult O. niloticus, phytoplanktons were dominant food items that constitute the bulk making the fish herbivorous. This is quite similar with the findings of (Assefa & Getahun, 2015; Teferi et al., 2000; Wagaw et al., 2022), that supported the herbivorous feeding practice of the fish. However, the highest amount of Cyanophyta reported in this study was in contrast to the findings of Engdaw et al. (2013) and Wagaw et al. (2022) who reported the preference and desire of the fish to diatoms. Variation in composition and relative contributions of food items may be explained by difference in microhabitat occupied by the fish, seasonal variation in sampling (Engdaw et al., 2013; Teferi et al., 2000; Wagaw et al., 2022); availability of algal growth limiting nutrients (Endgaw, 2020; Gao et al., 2017), fluctuation in water level and turbidity of the aquatic system.
It is a fact that, diet compositions of different food items consumed by O. niloticus changed as the fish grows older and increases with size. Juvenile O. niloticus mainly consume zooplanktons particularly, Rotifera, Copepods and Cladocerans. Although the nutritive quality of feeds from foods of animal origin is high and suitable during the early stages of the fish, the energy demand to fulfill the metabolic activity of adult fish cannot be attained through particulate feeding on zooplanktons and benthic invertebrates (Engdaw et al., 2013; Shipton et al., 2008). Therefore, attaining ontogenetic dietary shift from zooplanktivorous (omnivorous) feeding habit to phytoplanktivorous (herbivorous) is a must to satisfy its energy requirement. The observed shift in the feeding habit of O. niloticus may be ascribed with availability and seasonality of food items. Apart from the size based difference, it might also be due to the water type and productivity of the Lake.
In the present study, juvenile O. niloticus TL < 10 cm mainly feed on zooplanktons and insects; while adult fishes TL > 10 cm mainly feed on phytoplankton, detritus and macrophytes. The finding is in consensus with reports from different aquatic systems (Negassa & Prabu, 2008; Njiru et al., 2004; Teferi et al., 2000; Tesfaye et al., 2020). Despite their micro-habitat and availability of other food items, zooplanktivorous habit of juveniles might be associated with their smaller body size that cannot gulp the open water to filter phytoplanktons energetically and due to their smaller stomach that might not support big food items such as detritus and macrophytes.
Conclusion
There is close relationship on the morphometric parameters of O. niloticus with allometric growth pattern in Lake Tana indicating poor growth condition. O. niloticus feeds on wide varieties of food items and phytoplankton and detritus were major food items in the diet of the fish. Zooplanktons and macrophytes were of intermediate importance to the total diet of the fish. There is significant ontogenetic dietary shift in the feeding habit of the fish among different size classes revealing omnivorous feeding habit. Therefore, to have profitable and sustainable production, fishery management and monitoring measures must also consider availability of important food items for the diet of O. niloticus in Lake Tana.