Introduction
Frog farming in Vietnam has been developed since 2000, but mainly uses frog breeds imported from Thailand. Currently, frog products are mainly frozen and are exported to the US, EU, Hong Kong, etc., accounting for the remaining 30% of the domestic market. In the 2006–2011 period, Vietnam’s export of frog products increased to 724 tons (Le, 2012). The rapid growth in food production in recent years has progressively created residue waste and by-products. Some of fishery by-products, including scales, skins and offal have also been discarded in quantities over 20 million tons per year. Hence, agricultural waste has been treated by manual methods resulting in economic losses for the livestock industry (Caruso, 2015).
Bioactive peptides are molecules that have a variety of impacts on cells, including the ability to improve human health through promoting biological functions of the body (Perez Espitia et al., 2012). Many activities of biological peptides have been introduced such as antibacterial (Li et al., 2012), antioxidant (Power et al., 2013), anti-thrombotic (Khiari et al., 2014), anti-hypertension (Pokora et al., 2014) and immunomodulatory activities (Haney & Hancock, 2013). Because of these benefits, the commercialization of peptides pharmaceuticals increased from 14.1 million dollars in 2011 to 25.4 million dollars in 2018 and continues to prompt scientists to perform considerable study in this field. To date, approximately 60 peptide-based drugs have been identified, indicating the number of produced peptides may grow significantly in the near future (Fosgerau & Hoffmann, 2015).
Frog skin is a source of antibacterial peptides (AMPs), which are abundant and found naturally on earth (Di Grazia et al., 2015). According to statistics of the antimicrobial peptide database in the latest updated version (referred to as ADP3), the majority of AMPs (75%) are from animal origins, especially amphibians, which account for 38% of the 1,078 independent AMPs (Wang et al., 2016). The main groups of AMPs include dermaseptins, brevinin, palustrins, cathelicidans, esculentins, magainin, nigrocins, ranatuerins, temporins, ranalexins, japonicins and tigerinins; therefore, AMPs not only have antibacterial properties, but also have biological characteristics such as antioxidant, wound healing and toxin neutralizing properties as outlined in older publications (Varga et al., 2019). Besides AMPs, alkaloid is an outstanding biological compound with broad-spectrum and potent antibacterial activity found in Bufo gargarizans toads (China); recent alkaloids are also used to fight tumors and inhibit cell proliferation (Qi et al., 2011).
There have been various methods used to successfully isolate compounds from many frog skin samples, such as Sephadex G-50 column gel filtration chromatography, high-performance liquid chromatography (HPLC), reverse phase HPLC (RP-HPLC), thin layer chromatography or gas chromatography with contrast mass spectrometry, ... (Table 1). According to the above studies, compounds are mainly extracted from frog skin using a method of soaking frog skin samples in methanol or ethanol solution or separation buffer such as trifluoroacetic acid/water, phenylmethylsulfonyl fluoride, and sodium acetate to treated skins in preliminary steps. This process occurs before strain extraction to increase sequestration ability. Depending on the substances in different animals, the method will be other due to the biological structure of their skin. Therefore, we need to study more approaches that are appropriate for the research object.
| No | Methods | Species | Origin | Compounds | References | |
|---|---|---|---|---|---|---|
| Extraction | Measurement | |||||
| 1 | HPLC | Mass spectrometry FAB/MS with VG ZAB-2HF | Litoria genimaculata | Australia | Naculatin and maculatin 1.1 | (Rozek et al., 1998) |
| 2 | HPLC | Electron ray ionization quadrupole ion trap mass spectrometry (ESIMS) | Bombina variegata | Germany | Bomesin | (Marenah et al., 2004b) |
| 3 | HPLC reverse phase (RP-HPLC) | Mass spectrometry | Hylarana guntheri | Asia | Brevinin | (Conlon et al., 2008a) |
| 4 | RP-HPLC | Mass spectrometry | Xenopus laevis | Nigieria | Magainin | (Zasloff, 1987a) |
| 5 | RP-HPLC | Mass spectrometry | Rana tsushimensis | Japan | Stejneger | (Conlon et al., 2006) |
| 6 | RP-HPLC | Mass spectrometry | Rana areolata | American | Esculentin-1a | (Di Grazia et al., 2015) |
| 7 | RP-HPLC | Mass spectrometry | Leptodactylus syphax | Brazil | Syphaxin (Spx) | (Dourado et al., 2007) |
| 8 | Sephadex G-10 and G-50 column chromatography, RP-HPLC | Mass spectrometry | Bombina orientalis | Korea | Bombesin (Bbs-LS) | (Park et al., 1989) |
| 9 | Sephadex G-50 column, HPLC | Mass spectrometry | Rana nigromaculata | Korea | Nigrocin | (Park et al., 2001) |
| 10 | Thin layer chromatography, gas chromatography | Mass spectrometry | Dendrobatid | South America | Alkaloid | (Daly et al., 1978; Daly et al., 2002) |
Currently, abundant raw materials contain a lot of bioactive compounds from the frog skin that have been applied in biomedicine manufacturing to create valuable products (Ideia et al., 2020). Therefore, the aim of this study is to provide an overview of extraction methods and classification of bioactive compounds on the skin of frogs (Anura) to provide scientific data for the development of studies on biological compounds from frog skin. Moreover, the results of this review will contribute to reducing environmental pollution and supply more biologically active ingredients for the biomedical market in the future.
Classifying compounds isolated from frog skins and toad skins
The majority of the compounds extracted from frog skins are alkaloids or peptides such as brevinin, magainin and bombesin and are shown in Table 2.
| Group | Subgroups | Compounds | Species | Amount (nmol) | Origin | References |
|---|---|---|---|---|---|---|
| Alkaloid | Fatty acids |
Indolizidines Piperidines Pumiliotoxins Pyrrolizidines Quinolizidines Tricyclic |
Melano phryniscus | - | Argentina, Brazil, Paraguay, Uruguay | (Daly et al., 1984; Daly et al., 2004; Rodríguez et al., 2011) |
| Guanidine | Chiriquitoxin | Atelopus limosus | - | Panama | (Yotsu-Yamashita & Tateki, 2010) | |
| Saxitoxin | Atelopus varius | - | Peru | (Yotsu-Yamashita et al., 2004) | ||
| Tetrodotoxin | Atelopus ignescens | - | Ecuador | (Yotsu-Yamashita et al., 1992) | ||
| Atelopus peruensis | - | Peru | (Mebs et al., 1995) | |||
| Atelopus spurrelli | - | Colombia | (Yotsu-Yamashita & Tateki, 2010) | |||
| A. varius | - | Peru | (Yotsu-Yamashita & Tateki, 2010) | |||
| Atelopus oxyrhynchus | - | Venezuela | (Mebs & Schmidt, 1989, Yotsu-Yamashita et al., 1992) | |||
| Atelopus subornatus | - | Colombia | (Mebs et al., 1995) | |||
| Atelopus limosus | - | Ecuador | (Yotsu-Yamashita & Tateki, 2010) | |||
|
Zetekitoxin AB Zetekitoxin-C |
Atelopus zeteki | - | Panama | (Brown et al., 1977; Yotsu-Yamashita et al., 2004) | ||
| Bombesin |
Bombesin-LS Bombesin-14 |
Bombina orientalis | - | Korea | (Park et al., 1989) | |
|
Bombesin H1 Bombesin H2 Bombesin H3 Bombesin H4 |
Bombina maxima | - | China | (Lai et al., 2002) | ||
| BR-Bombesin | Boana raniceps | - | Argentina | (de Sousa et al., 2022) | ||
| PR-Bombesin | B. maxima | - | Asian | (Li et al., 2006) | ||
| Brevinin | Brevinin 1 | Brevinin-1P | Odorrana schmacker | - | China | (Chen et al., 2006) |
| Brevinin-1S | Odorrana versabilis | - | China | (Chen et al., 2006) | ||
| Brevinin-1V | Pelophylax plancyi fukienensis | - | China | (Chen et al., 2006) | ||
| Brevinin-1E | Rana esculenta | - | China | (Kumari & Nagaraj, 2001) | ||
| Brevinin-1CBa | Lithobates catesbeianus | - | Canada | (Mechkarska et al., 2011) | ||
| Brevinin-1CBb | L. catesbeianus | 165 | Canada | (Mechkarska et al., 2011) | ||
| Brevinin-1Lb | Rana luteiventris | 45 | Colombia | (Goraya et al., 2000) | ||
| Brevinin-1Ba | Rana berlandieri | 4 | (Goraya et al., 2000) | |||
| Brevinin-1Bb | R. berlandieri | 15 | America | (Goraya et al., 2000) | ||
| Brevinin-1Bc | R. berlandieri | 8 | America | (Goraya et al., 2000) | ||
| Brevinin-1Bd | R. berlandieri | 28 | America | (Goraya et al., 2000) | ||
| Brevinin-1Be | R. berlandieri | 12 | America | (Goraya et al., 2000) | ||
| Brevinin-1Bf | R. berlandieri | 9 | America | (Goraya et al., 2000) | ||
| Brevinin-1Pa | R ana pipiens | 1,050 | Canada | (Goraya et al., 2000) | ||
| Brevinin-1Pb | R. pipiens | 1,110 | Canada | (Goraya et al., 2000) | ||
| Brevinin-1Pc | R. pipiens | 64 | Canada | (Goraya et al., 2000) | ||
| Brevinin-1Pd | R. pipiens | 315 | Canada | (Goraya et al., 2000) | ||
|
Brevinin-1Sa Brevinin-1Sb |
R. berlandieri | - | America | (Conlon et al., 2004b) | ||
| Brevinin-1Sc | R. pipiens | - | Canada | (Conlon et al., 2004b) | ||
|
Brevinin-1LTa Brevinin-1LTb |
Hylarana latouchi | - | China | (Wang et al., 2009) | ||
|
Brevinin1RTa Brevinin1RTb Brevinin-1RTc |
Amolops rickettii | - | China, Vietnam | (Wang et al., 2012) | ||
| Brevinin 2 | Brevinin-2R | Rana ridibunda | - | Europe | (Ghavami et al., 2008) | |
| Brevinin-2GUb | Hylarana guntheri | - | China | (Conlon et al., 2008a) | ||
| Brevinin-2(B2RP) | Lithobates septentrionalis | - | America | (Abdel-Wahab et al., 2010) | ||
|
Brevinin-2RTa Brevinin-2RTb |
A. rickettii | - | China, Vietnam | (Wang et al., 2012) | ||
| Brevinin-2PRa | Rana pirica | 680 | Japan | (Conlon et al., 2004a) | ||
| Magainin |
Magainin 1 Magainin 2 |
Xenopus laevis | - | Nigieria, Africa | (Edelstein et al., 1991; Lai et al., 2002) | |
|
Magainin A Magainin G |
B. maxima
Bombina variegata |
- | China, Germany | (Balboni et al., 1992; Edelstein et al., 1991; Lai et al., 2002; Ohsaki et al., 1992) | ||
| Other compounds |
Adenoregulin Dermaseptin I Dermaseptin B Dermatoxin Phylloseptins |
Phyllomedusa | - | South America | (Amiche et al., 1993; Amiche et al., 2000; Brand et al., 2002; Leite et al., 2005; Mor & Nicolas, 1994) | |
| Bomesin | B. variegata | - | Europe | (Marenah et al., 2004b) | ||
|
Cruzioseptin-16 Cruzioseptin-17 |
Cruziohyla calcarifer | - | Costa Rica | (Sousa et al., 2009) | ||
|
Dermorphin Sauvagine Dermaseptin I |
Phyllomedusa sauvagei | - | South America | (Montecucchi et al., 1981; Mor & Nicolas, 1994) | ||
| Esculentin-1 | Odrrana hossi | - | Japan | (Conlon et al., 2008b) | ||
| Esculentin-1a | Rana areolata | 395 | America | (Di Grazia et al., 2015) | ||
|
Esculentin-2 Nigrocin-2 |
Hylarana pictureurata | - | Malaysia | (Conlon et al., 2008b) | ||
|
Esculentin-1 Temporin-1 Palustrin-2 Palustrin-3 Ranatuerin-2 |
R. areolata | - | United States | (Ali et al., 2002) | ||
|
Esculentin-2P Octadecapeptidep-LR Ranateurin-2P |
R. pipiens | - | Canada | (Chinchar et al., 2001; Manika et al., 1998) | ||
| Gaegurins Rugosins | Rana rugosa | - | Japan | (Park et al., 1994, Suzuki et al., 1995) | ||
|
Japonicin-1 Japonicin-2 Temporin-1Ja |
Rana japonic | - | Japan | (Isaacson et al., 2002) | ||
|
Maculatin 1.1 Naculatin |
Litoria genimaculata | - | Australia | (Rozek et al., 1998) | ||
|
Nigrocin 1 Nigrocin 2 |
Rana nigromaculata | - | East Asia | (Park et al., 2001) | ||
|
Ocellatin-1 Ocellatin-4 |
Leptodactylus | - | South America | (Sousa et al., 2009) | ||
| Phylloseptin-L2 | Hylomantis lemur | 95 | Costa Rica | (Abdel-Wahab et al., 2008) | ||
| Plasticin-L1 | Leptodactylus laticeps | 130 | South America | (Sousa et al., 2009) | ||
|
Proline -arginine Rhinophrynin-27 |
Rhinophrynus dorsalis | - | Mexico | (Carta et al., 2021) | ||
| Ranatuerins 1-9 | Rana catesbeiana | - | Japan | (Goraya et al., 1998) | ||
| Ranalexin | R. catesbeiana | - | America | (Clark et al., 1994) | ||
| Rohdei-litorin | (Montecucchi et al., 1984; Renda et al., 1985) | |||||
| Stejneger | Rana tsushimensis | - | Japan | (Conlon et al., 2006) | ||
|
Syphaxin (Spx) Syphaxin 1,5 |
Leptodactylus syphax | - | Brazil | (Dourado et al., 2007) |
Alkaloid is a natural compound constituted by the basic element nitrogen together with carbon, hydrogen, oxygen, sulfur and some other rare elements such as chlorine, bromine and phosphorus (Babbar, 2015). Alkaloids are found on the skin of amphibians such as lipophilic alkaloids, indol alkaloids and guanidine alkaloids. Alkaloid contents are mostly found on frog skin, and it has been proposed that they occur due to accumulation from preferred food sources like arthropods (Bolton et al., 2017; Hovey et al., 2018).
In general, there are two kinds of alkaloids that are classified based on the distribution of subgroups; the first group involves in a fatty acid tag, whilst the second contains a guanidine group. Both alkaloid groups have only been extracted in entirety from kinds of frogs that live in some countries of South America including Argentina, Brazil, Paraguay, Uruguay, Panama, Peru, Colombia, Venezuela and Ecuador. Guanidine alkaloids are commonly found on the Atelopus genus of Anura (Daly et al., 2008; Yotsu-Yamashita et al., 1992). Although these alkaloids were isolated mainly from the skin of Atelopus frogs, they have not been found or have very little presence on other parts of the frog (Kim et al., 1975). These characteristics of frog skin are considered a poison for frog species that can instantly destroy their enemies, especially carnivores (Daly et al., 1987; Kim et al., 1975). With the exception of Melano phryniscus, which showed the presence of five components of alkaloids with fatty acids, and Atelopus zeteki, which contained the two toxins Zetekitoxin-AB and Zetekitoxin-C (Brown et al., 1977; Kim et al., 1975; Shindelman et al., 1969), each of the nine remaining species contained one component of alkaloid. As shown in Table 2, a toxin like saxitoxin was found on the skin of Atelopus varius and its structure was similar to alkaloid 1 (Yotsu-Yamashita et al., 2004). The four species A. varius, Atelopus oxyrhynchus, Atelopus subornatus and Atelopus spumarius all had a single tetrodixin, although they come from differential countries (Mebs et al., 1995; Mebs & Schmidt, 1989; Yotsu-Yamashita et al., 1992; Yotsu-Yamashita & Tateki, 2010). Finally, the chiriqui toxin was harvested from the skins of Atelopus limosus (Yotsu-Yamashita & Tateki, 2010).
The most prevalent antimicrobial peptide in the skin of amphibians is brevinin (Wang & Wang, 2004), which is attracting attention in medical fields, especially in the development of pro-drugs for the backbone of first-line defences against various microorganisms (Savelyeva et al., 2014) (Table 2). They are straight, amphoteric, cationic, cyclic heptapeptides with a disulfide bridge at the C-terminus (Clark et al., 1994). Currently, there are about 350 different types of brevinin identified (according to DADP). There are two main groups of brevinin, brevinin-1 and brevinin-2, which are isolated mainly from the species Rana brevipodaporsa (Morikawa et al., 1992), but are also found in Rana okinavana and Ranaseptentrionalis (Conlon et al., 2005a; Conlon et al., 2005b). As for the Ranidae family, their antimicrobial peptides are divided into 14 groups, of which the two main groups are brevinin-1 and brevinin-2 (Conlon, 2008). In the brown frog Rana tsushimensis (Tsushima) there are six AMPs of the brevinin group (Conlon et al., 2006). Brevinin-1 is also present in Rana areolata (Ali et al., 2002) and in Rana pirica (Hokkaido, Japan). There are five AMPs belonging to the brevinin-2 group.
The brevinin compounds on amphibian skins have shown diversity and abundance. The most prominent exist on the genus Rana in the family Ranidae, which accounts for more than half of the brevinins in the Anura species. All distributions of the differential components are shown in Table 2. The peptide class composed of 24 amino acids in length with a conserved amino acid proline at the 14th position of the N-terminus and a Ranabox is called the brevinin-1 group (Table 3). Based on the homology characteristics of structural sequences, many components were gathered into nine subgroups. The first results showed derivations including brevinin-1P, brevinin-1S, brevinin-1V and brevinin-1E, which have been collected from the four species of Chinese frogs Odorrana schmacker, Odorrana versabilis, Pelophylax fukienensis and Rana esculenta, respectively (Chen et al., 2006; Kumari & Nagaraj, 2001). As an individual subgroup, a single brevinin-1Lb compound presented in Rana luteiventris of Canada, and two derived brevinin-1CBa and brevinin-1-CBb compounds were only found in dissimilar Lithobates catesbeianus species in Canada. Also, the complex distributions of many components with homologous structures have separated due to classification; hence, these components were arranged and classified into the larger subclasses such as Brevinin-1B or Brevinin-1P. Specifically, both of these larger subclasses have been isolated from Rana berlandieri of the U.S. and Rana pipiens of Canada. Each subclass had four or five components with their contents have measured obviously. Five components of the Brevinin-1B subclass have isolated contents obtained from 4 nmol to 45 nmol while the extracted contents of the Brevinin-1P subclass had higher concentrations ranging from 64 nmol to 1,110 nmol (Goraya et al., 2000). The remaining compounds were divided into two larger classes; a variety of AMPs such as the peptides brevinin-1RTa, brevinin-1RTb, brevinin-1RTc, brevinin-2RTa and brevinin-2RTb were found in Amolops rickettii (Wang et al., 2012) and peptides that were likely Brevinin-1Sa, Brevinin-1Sb and Brevinin-1Sc were recorded in the two species R. berlandieri (America) and R. pipiens (Canada). There is also brevinin-2GUb from Hylarana guntheri (Conlon et al., 2008a), brevinin-2 (B2RP) on Lithobates septentrionalis (Abdel-Wahab et al., 2010) and brevinin-2Pra on R. pirica (Conlon et al., 2004a). On the skin of the Hokkaido frog R. pirica, isolated brevinin-2PRa was refined to the amount of 680 nmol/g dry weight of frog skin. In addition to antimicrobial peptides, there are insulin-releasing peptides of the brevinin group including brevinin-2GUb from H. guntheri (Conlon et al., 2008a; Daly et al., 1994), brevinin-2-related peptide (B2RP) from L. septentrionalis (Abdel-Wahab et al., 2010; Mebs et al., 1995) and the peptide brevinin-1 from Lithobates palustris (Marenah et al., 2004a; Wang et al., 2016), Pelophylax saharicus (Marenah et al., 2006; Yotsu-Yamashita & Tateki, 2010) and Glandirana emeljanovi (Kim et al., 2010).
| Structure name | Sequence/structure | Active | References | |
|---|---|---|---|---|
| Alkaloid | Alkaloid guanidine: Zetekitoxin-AB |
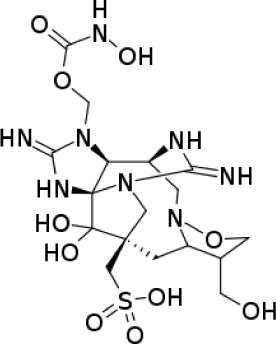
|
Antibacterial activity of Escherichia coli MIC is 10 μg/mL | (Yotsu-Yamashita et al., 2004) |
| Alkaloid indol: bufotenine | No | Anti-tumor activity | (Renda et al., 1985) | |
| Lipophilic alkaloids: Pyrrolizidine |
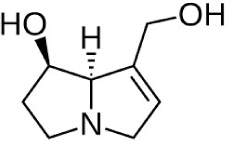
|
The MIC antibacterial activity was 3.9 μg/mL against Staphylococcus aureus and 31.3 μg/mL against E. coli | (Daly et al., 2005) | |
| Bombesin | Bombesin-likes substances (Bbs-LS) | No | Stimulates the release of the stomach hormone gastrin | (Park et al., 1989) |
|
Bbs-14 Synthetic RP- Bombesin |
GGNQWAIGHFM-NH2 | Antibacterial activity of S. aureus with MIC of 25 μg/mL, stimulating the release of gastric hormone gastrin | (de Sousa et al., 2022) | |
| Brevinin | Brevinin 1 | FLPVLAGIAAPALFCKITKKC | Antibacterial activity of E. coli with MIC of 26 μg/mL, stimulates insulin release | (Conlon et al., 2008b) |
| Brevinin 2 | GLLDSLKGFAATAGKGVLQSLLSTASCKLAKTC | |||
| Brevinin-2GU | GVIIDTLKGAAKTVAAELLRKAHCKLTNSC |
Significantly reduces the production of TNF-α from concanavalin A (Con A)-stimulated PBM cells Suppression of IFN- production from unstimulated PBM cells |
(Popovic et al., 2012) | |
| Magainin | Magainin 1 | GIGKFLHFAGKFLKAFVLEIMKF | Resistance to E. coli D31 with MIC of 5 μg/mL and S. aureus MIC of 50 μg/mL | (Zasloff, 1987a) |
| Magainin 2 | GIGKFLHFAKKFLKAFVLEIMNF | |||
| Magainin A | AIGKFLHAAKKFAKAFVAEIMNF | Anticancercontraceptive, antifungal and antiviral activity | (Ohsaki et al., 1992) | |
| Magainin G | GIGKFLHSAGKFGKAFVGEI | |||
| Other compounds | Cruzioseptin-16 | GFLDVLKGVGKAALGAVTHLINQGEQ | Resistant to bacteria | (Cuesta et al., 2021) |
| Cruzioseptin-17 | GFLDVLKGVGKAALGAVTHLINQGEQ | |||
| Esculentin-1a | GIFSKLAGKKIKNLLISGLKG-NH2 | Stimulation of HaCaT cell migration and gram-negative antibacterial activity. Pseudomonas aeruginosa | (Di Grazia et al., 2015) | |
| Ocellatin-4 | GLLDFVTGVGKDIFAQLIKQI-NH2 | Resistant to bacteria | (Nascimento et al., 2004; Nascimento et al., 2007) | |
| Phylloseptin-L2 | FLSLIPHVISALSSL | Stimulates insulin release | (Abdel-Wahab et al., 2008) | |
| Plasticin-L1 |
GLVNGLLSSVLGGGQGGGGL LGGI-Xaa |
Stimulates insulin release | (Conlon et al., 2009) | |
| Proline-arginine | ELRLPEIARPVPEVLPARLPLPALPRN | Stimulates insulin release | (Carta et al., 2021) | |
|
Rhinophrynin-27 Syphaxin |
No | Resistant to bacteria | (Dourado et al., 2007) |
In the membrane, magainin is an amphiphilic peptide with a length of 21–26 residues that form an α-helical structure. According to several studies, magainin is usually found mainly in amphibians (Tables 2 and 3). A family of four magainins are found in the skin of the Bombina genus and Xenopus laevis. The group of magainins are magainin A (Mag A) and magainin G (Mag G) (Balboni et al., 1992; Edelstein et al., 1991; Lai et al., 2002; Ohsaki et al., 1992), which are commonly found in Bombina maxima of China and Bombina variegata of Germany. Another species such as X. laevis (Nigieria, Africa) also synthesizes many magainin 1 and magainin 2 peptides, which were determined to contain 23 amino acid residues but different amino acid positions 10 and 22, hence the general sequence of magainin 1 and magainin of GIGKFLHFAG/KKFLKAFVLEIMK/NF with positions 10 and 22 having the magnetic weak substitution of Glycine and Lysine in Mag 1 with Lysine and Asparagine in Mag 2 (Zasloff, 1987). The synthesis of the above results shows that the discovery of subtypes of magainin compounds is a first achievement for the identification of potential peptide compounds extracted from amphibian skin.
Bombesin is a peptide consisting of 14 residues also known as a tetradeca peptide, which was first isolated from Bombina bombina in 1970 in Korea. Together with ranatensin and phyllolitorin, bombesin have been discovered in the skin of species including Bombina, Rana and Phyllomedusa. Two kinds of bombesins, bombesin-LS and bombesin-14, have been extracted in methanol from the skin of Bombina orientalis in Korea, while they isolated four subtypes, bombesin 1, bombesin 2, bombesin 3 and bombesin H peptides, which the scientists found in the same B. orientalis in China (Lai et al., 2002). In addition, a peptide involves antimicrobial activity and proline-rich PR-Bombesin has also been isolated from B. maxima (Chinese) (Li et al., 2006) and another BR-Bombesin contains the amino acid sequence GGNQWAIGHFM-NH2 (Table 3) and was harvested on Boana raniceps in Argentina (de Sousa et al., 2022). All components of bombesin are decribed in the Table 2.
Biological compounds are often found on frog skin, but each continent has its own compounds that have been synthesized and are shown in Table 2. For Asian countries, Japan is considered a country have many biological compounds. The first is the compound esculentin-1 extracted from the skin of Odrrana hossi (Conlon et al., 2008b), Japonicin-1, Japonicin-2 and Temporin-1Ja are found in Rana japonic (Isaacson et al., 2002), R. tsushimensis contains Stejneger (Conlon et al., 2006), Rana rugosa contains Gaegurins and Rugosins (Park et al., 1994; Suzuki et al., 1995) and nine peptides of Ranatuerins 1–9 were found in Rana catesbeiana (Goraya et al., 1998). In addition, in Malaysia, Hylarana pictureurata was found to contain Esculentin-2 and Nigrocin-2 (Conlon et al., 2008b), and Nigrocin-2 was also found in the Asian frog Rana nigromaculata, which also contained Nigrocin-1 (Park et al., 2001). Compounds in Asia are still largely unknown. For Americas countries, as America is considered the home of amphibians by many bioactive compounds have been found on the skin of amphibians such as Esculentin-1a found in R. areolata with a weight of 395 nmol (Di Grazia et al., 2015) and Ranalexin in R. catesbeiana are all in America (41). In Brazil, Syphaxin (Spx) and Syphaxin 1.5 were found in Leptodactylus syphax (Dourado et al., 2007) and Ryptophyllin (FPPWM-NH2) and Rohdei-litorin in Phyllomedusa rhodei (Barra et al., 1985; Montecucchi et al., 1984; Renda et al., 1985). Or in South America, 130 nmol Plasticin-L1 was found in Leptodactylus laticeps (Sousa et al., 2009), mixed Ocellatin-1 and Ocellaltin-4 in Leptodactylus (Sousa et al., 2009), Phyllomedusa sauvagei contains Dermorphin, Sauvagine and Dermaseptin I (Montecucchi et al., 1981; Mor & Nicolas, 1994) and a variety of substances such as dermaseptin I, dermaseptin B, dermatoxin, DS01, adenoregulin (with 33 amino acids) and PS-1 (Phylloseptins) were found in the Phyllomedusa family (Amiche et al., 1993; Amiche et al., 2000; Brand et al., 2002; Leite et al., 2005; Mor & Nicolas, 1994). The most prominent ones are octadecapeptidep-LR, esculentin-2P (E2P) and ranateurin-2P (R2P) isolated from R. pipiens (Canada) (Chinchar et al., 2001; Manika et al., 1998). The norepinephrine-stimulated Hylomantis lemur species produces a peptide from the Phylloseptin family Phylloseptin-L2 with a mass of 95 nmol (Abdel-Wahab et al., 2008). Cruzioseptin-16 and cruzioseptin-17 were found in the skin of the frog Cruziohyla calcarifer, which belong to a new family of 15 antimicrobial peptides named cruzioseptins, both from Costa Rica (Sousa et al., 2009). Particularly, as R. areolata in America contains brevinin-1 as well as many other substances such as temporin-1, palustrin-2, palustrin-3, esculentin-1 (two peptides) and the Ranatuerin-2 family (two peptides) (Ali et al., 2002). In addition, a mixed group of the two compounds proline-arginine and rhinophrynin-27 (RP-27) has been isolated from the skin of Rhinophrynus dorsalis (Carta et al., 2021). Moreover, the presence of biological compounds also detected on the skin of amphibians in some European countries and Oceania. For example, the skin of Litoria genimaculata (Australia) contains naculatin and maculatin 1.1 (Rozek et al., 1998). The Bomesin group was extracted from the skin of the toad B. variegata (Germany) (Marenah et al., 2004b). Based on the peptide components and identified compounds, indicating an abundance of substances in nature.
Biological activities of compounds isolated from frog skins
The most prominent activities have been identified as antibacterial, antifungal, anti-tumour and stimulating insulin production were shown in Table 3.
The class of alkaloids and its biological activity were evaluated as early as the 18th century. Overall, three subtypes of alkaloids, including alkaloid lipophilic, alkaloid guanidine, and alkaloid indole, were determined to have antibacterial activities against bacterium after 24 hours incubation. The appearance of a sterile ring was used to evaluate the bacterial growth inhibition zone (Preusser et al., 1975). Among the three subtypes, pyrrolizidine made the properties of the alkaloid lipophilic and showed antibacterial activity against both Staphylococcus aureus and E scherichia coli bacteria with microorganism inhibitory concentration (MIC) values of 3.9 μg/mL and 1.3 μg/mL, respectively. The antibacterial activity of the alkaloid guanidine constituted by zetekitoxin-AB was against E. coli bacteria with a MIC of 10 μg/mL (Daly et al., 2005; Yotsu-Yamashita et al., 2004). Finally, the bufotenine that are extracted from the skin of B. gargarizans have a modification of the indol group and therefore inherited additional anti-tumour activities (Renda et al., 1985). In addition, the activity of the other compounds extracted from the skin of B. gargarizans exhibit significant anti-tumor activities including cytotoxic effects such as inhibiting cell proliferation, interrupting the cell cycle, inducing apoptosis, inducing cell differentiation and inhibiting angiogenesis (Qi et al., 2011). Hence, there are many types of alkaloids with good benefits to be extracted from skin of amphibians.
Brevinin is one of the common antimicrobial peptides, including brevinin-1 and brevinin-2 which were recovered from Rana brevipoda porsa. These two groups have been determined antimicrobial activity against two pathogenic bacteria including S. aureus (ATCC 25923) and E. coli (ATCC 2592), and the same MIC value of 26 μg/mL was mentioned for both bacteria (Conlon et al., 2008b) (Table 3). Furthermore, its antibacterial properties have revealed effects relating to insulin release. For example, to evaluate the impact on insulin release a chemically synthesized brevinin-1 product demonstrated an inducing ability to release insulin in BRIN-BD11 cells from 1.5 to 3.5 times after this cell passed inoculated on RPMI-1640 medium, and identified additional thresholds of effective brevinin-1 values ranging from 10–8 to 10–6 M. In contrast, lower threshold concentrations of brevinin-1 resulted in abrogation of insulin-releasing activity in BRIN-BD11 cells. This is the first published work to demonstrate that frog skin secretions contain a peptide class that inherits insulin-releasing activity. Because amphibians may produce large amounts of peptides in their secretions for defence, this class of peptides can prevent attacks by promoting the release of insulin and producing hypoglycemia in the skin of predator animals (Marenah et al., 2004a). Insulin release is noticeable, but brevinin has also demonstrated an effect on cytokine release. In the presence of the Brevinin-2GU peptide relating to concanavalin-stimulated peripheral blood mononuclear cell (PBM) cell cultures showed an increased release of proinflammatory and anti-inflammatory cytokines compared with the control PBM cell line without the addition of this peptide (Popovic et al., 2012). The synthesis of brevinins on the skin of amphibians shows a diversity and abundance of biological activities, which can be used to support the treatment of many diseases.
Although magainin 1 and magainin 2 have a general sequence of 20 amino acids (GIGKFLHSAGKFG KAFVGEI) with highly homologous identities except magainin G1, which ends with the amino acid sequence GEIM and magainin G2, which ends with the sequence GILN (Table 3). However, only Magainin 1 isolated from the skin of X. laevis has been considered an extremely toxic substance for both bacteria such as E. coli and Saccharomyces cerevisiae and some other gram-negative bacteria including Candida albicans, S. cerevisiae and Cryptococcus neoformansand, and it also induces osmotic lysis in some protozoa (Zasloff, 1987). In particular, the antibacterial activities of E. coli D31 have a MIC of 5 μg/mL and S. aureus have a MIC of 50 μg/mL (Zasloff, 1987). According to the results of the evaluation of antibacterial activity, magainin has been declared an extremely beneficial peptide for humans. Also, other magainins such as magainin A (Mag A) and magainin G (Mag G) extracted from frog skin of B. variegata in Germany were identified as having anti-tumour activities against lung cancer cells, inhibit the proliferation of human leukemia, have anti-bacterial, anti-viral, anti-fungal and spermicidal properties and can be used as contraception against sexually transmitted infections except HIV-1 and HIV-2 (Edelstein et al., 1991; Lai et al., 2002). In vitro anti-tumor efficacy of Mag A and Mag G against SCLC cell lines was demonstrated by the experiment’s results (Ohsaki et al., 1992). The synthesis of the above results shows that the discovery of subtypes of magainin compounds is the first achievement for the identification of potential peptide compounds extracted from amphibian skin.
These peptide groups (bombesins) are distinguished by their receptor-selective responses and the sequence of amino acids attached to their C-terminus. The single bombesin group at the C-terminus is attached to the GHLM-NH2 chain, while ranatensins are terminated with the GHFM-NH2 sequence and the C-terminal phyllolitorin is the GFF/LM-NH2 sequence (Lin et al., 2017). The biological activity of BR-bombesin was investigated in the stomach and esophagus of Wistar rats, and the results showed that BR-bombesin inhibited gastric secretion by about 50%. This novel peptide has 80% and 70% similarity with the C-terminal region of human neuromedin B (NMB) and the human gastrin-releasing peptide (GRP10), respectively. Molecular binding analysis showed that the GRP receptor has binding energies of –7.3 kcal.mol–1 and –8.5 kcal.mol–1, respectively, when interacting with bombesin and BR-bombesin. It is therefore concluded that BR-bombesin has a strong impact on gastrointestinal disorders and gastric acid secretion. In addition, the antibacterial level of this peptide was evaluated on S. aureus with a MIC of 25 μg/mL (de Sousa et al., 2022). In addition, in European frogs raised in Korea, the biological activity of purified Bombesin-likes substance (Bbs-LS) was tested and compared with that of synthetic Bbs-14, and it was found that both substances have gastric hormone-releasing activity of thick gastrin. Bombesin and bombesin derivatives are found only in amphibians. Therefore, researchers have now conducted a bombesin study focusing on six species of Bombina to bring out the best benefits.
Currently, the two main classes of biological activity derived from amphibian skin extracts are antimicrobial and insulin release stimulators. The proline-arginine mixture Rhinophrynin-27 (RP-27) isolated from the skin of R. dorsalis was able to stimulate insulin release from the rat pancreatic β-cell line BRIN-BD11 (Carta et al., 2021). For the B. variegata skin-extracted Bomesin group, the results showed a 1.5–3.5-fold increase in insulin release after acute incubation with glucose-responsive BRIN-BD11 cells compared with 5.6 mM glucose that alone showed potent insulin release enhancing activity from pancreatic BRIN-BD11 cells (Marenah et al., 2004b). Not only that, a glycine-leucine-rich peptide called Plasticin-L1 has been shown to accelerate insulin release by glucose-responsive pancreatic β-cells BRIN-BD11 without accelerating lactate releasing dehydrogenase (Conlon et al., 2009). The most prominent was phylloseptin-L2, which demonstrated the ability to induce significant stimulation of insulin release from BRIN-BD11 cells at 30 nM, with a maximal response at 3 μM. At doses up to 3 M, phylloseptin-L2 did not induce cytosolic lactate dehydrogenase enzyme release, demonstrating that plasma membrane integrity is maintained. Significantly more total insulin was secreted and enhanced glucose tolerance in rats was shown after receiving phylloseptin-L2 (50 nmol/kg b.w.) following intraperitoneal glucose (18 mmol/kg b.w.) (Abdel-Wahab et al., 2008). The second is the antibacterial activity group, and through the test results, Cruzioseptins has an antibacterial effect against E. coli, C. albicans, S. aureus and low hemolytic efficiency, in which Cruzioseptin-16 and Cruzioseptin-17 had the strongest antibacterial activity (Cuesta et al., 2021). Next, the ocellatin group was determined to be active against E. coli, the ocellatin-4 survey showed antibacterial activity against both E. coli and S. aureus and all MICs were equal with the same value of 64 μM (Nascimento et al., 2004; Nascimento et al., 2007). In addition, the truncated Syphaxin (Spx) fragments, Spx (1–22) and Spx (1–16), were tested for their activity against E. coli and S. aureus, showing low MICs and no significant hematopoietic toxicity (Dourado et al., 2007).
The most impressive activity was over a wide range of peptide concentrations (0.025–4 M), the biological activity of Esculentin-1a (1–21) NH2 strongly stimulated migration of immortalized human keratinocytes (HaCaT cells), and this was especially more effective in human cathelicidin (LL-37). This peptide has a wide range of antimicrobial activities with proven efficacy against both the plankton and biofilm forms of gram-negative bacteria (Di Grazia et al., 2015). Until now, biological compounds were found in an amphibian, indicating an abundance of substances in nature.
Conclusion
This review provides a wealth of new, isolated valuable sources of bioactive compounds on frog skins come from in various countries in the world. Components such as guanidine alkaloid, brevinin 1&2, brevinin-2GU, magainin 2, magainin A&G have also demonstrated antibacterial, antifungal, and antiviral properties. Two other members, lipophilic alkaloid and magainin 1 gave the highest antibacterial activities. In addition, while Bbs-LS could stimulate the release of the gastric hormone gastrin, the other peptide groups such as brevinin-1, phylloseptin-L2, plasticin-L1, bomesin, or phylloseptin-L2 revealed the ability to promoting insulin release. Thereout, two special components are bufotenine and brevinin-2GU showed other highlighted features relating to anti-tumor activity, reducing the production of TNF-α. In conclusion, an overview of found biological compounds in frog skins in this study shows that extracts from frog skin bring many benefits to humans. These science-based findings set the stage for more extensive scientific studies.
