Introduction
The Nile tilapia (Oreochromis niloticus Linnaeus 1758), the African catfish (Clarias gariepinus Burchell 1822), and the African big barb (Labeobarbus intermedius Rüppell 1835) were commercially important fish species in the Ribb Reservoir, Tana basin, South Gondar, Ethiopia. Fish biometrics, such as the length-weight relationship, the length-length relationship, and conditions factors, are widely used methods of fisheries management. They provide biological evidence of the health of the fish, growth patterns, ontogenetic changes, and the dynamics of the fish stock (Kebede et al., 2018). The relationship length-weight is crucial to the estimation of weight based on length measurements. In addition, the relationship between length and weight is important to calculate the assessment of fish stocks through the development of the assessment model of fish stocks (Tesfaye et al., 2016).
In particular in developing countries, fish reproductive biology is significant in the fisheries management strategy. According to Tesfaye et al. (2016) notified that fishery managers should strictly follow size at first maturity and close the spawning season to sustain fishery practices. Otherwise, the fisheries would be over-exploited and inadequately managed.
In river ecosystems, water quality parameters are influenced by dams, which in turn affects fish reproduction and behavior (Anteneh et al., 2013). Changes in water temperature and river hydrodynamics have adverse effects on many freshwater fish species’ reproductive performance and other biological parameters (Adams et al., 2015). For example, cold water temperatures below the reservoir can affect the development of the ovarian follicle, the diameter of the ovarian follicle, and the reproduction rate of fish species. In reservoirs, the reproductive performance of migratory fish and non-migratory fish was negatively affected by temperature and dissolved oxygen in the summer due to thermal stratification (Mequanent et al., 2021). In addition, fertility is considered a biological parameter closely related to the availability of food, physical and chemical variables in water.
In Lake Tana, 17 Labeobarbus fish species were found (Mequanent et al., 2021). Of these, seven species, namely Labeobarbus acutirostris, Labeobarbus brevicephalus, L. intermedius, Labeobarbus megastoma, Labeobarbus macrophtalmus, Labeobarbus truttiformis, and Labeobarbus tsanensis are migratory species. During the rainy season, these migratory breeding species can travel more than 60 km to tributary rivers and streams, to access better breeding grounds in July through October (Anteneh et al., 2013). These fish species require rapid, clear, well oxygenated river or river with gravel bed for the success of reproduction. However, nowadays the spawning grounds (rivers) are in increasing peril from anthropogenic activities. For example, the Ribb Dam (73 m high) and irrigation weirs have blocked the migratory spawning species of Labeobarbus (Mequanent et al., 2021). The fishes could not jump to migrate upstream without a fish ladder (corridor) to find a favorable environment for spawning purposes. In line with this, Anteneh et al. (2013) and Getahun et al. (2008) pointed out that the weirs or dam with fish ladder are important particularly for the migratory spawning Labeobarbus species from Lake Tana to Ribb River. However, the fish ladder is not included. For this reason, our study was focused on the riverine origin fish species above the dam including O. niloticus, C. gariepinus, and one Labeobarbus (L. intermedius) species that probably which is isolated during damming.
The Ribb River is one of the rivers that feed Lake Tana. Ribb Reservoir is new and its construction is completed recently the fisheries activity also started in 2018. The reservoir also plays an important role in fisheries and irrigation practices, as well as in the livelihoods of the nearby communities. Very few studies have been conducted in the Ribb River about the effects of dams and dams on the migration of reproductive types of the Labeobarbus species (Anteneh et al., 2013; Getahun et al., 2008) and the impact of irrigation practices on fishing activities (Mequanent et al., 2021). However, there has been no study of the biological and reproductive aspects of O. niloticus, C. gariepinus and L. intermedius in the Ribb Reservoir. Therefore, the purpose of this study was to conduct some biological aspects and reproductive biology of commercially important fish species in the reservoir to address the scientific gap for better management of fish resources to sustain fisheries.
Materials and Methods
The Ribb Dam has recently been constructed and new fishing activity has been established in 2018. The reservoir has three inflow rivers (Melo, Ribb, and Hamus). The Ribb Dam is found in the Ribb River in the South Gondar Zone of the East side of the sub-basin of Lake Tana, Amhara region. The Ribb River is about 130 km long, its drainage area is almost 1,790 km2, and the average annual discharge is 14.6331 m3/s (Bezabih, 2021). Ribb Dam is neighbored by four kebeles including Medeb Gubda, Jarashikra, and Ayvaniva from Farta woreda and Amstya villages from Ebnat district. It has an area of about 685 km2 in the dam. According to the Universal Transverse Mercator (UTM), Ribb Dam is located in the North-Western part of Ethiopia, by the geographic coordinate of 12° 02’ 30” N and 37° 59’ 45” E at altitudes ranging from 1,880 to 1,970 m. The climate in the Ribb basin is characterized by a rainy season that begins from May to October. Monthly rainfall ranges from 65 mm in May to 411 mm in July. The annual average rainfall is about 1,400 mm, with the lowest precipitation around 1,200 mm. During the year, temperatures range from 19°C in December to 23°C in May, with maximum temperatures of 30°C and minimum temperatures of 11.5°C. The wind speed is low and the possible amount of evapotranspiration from December (95 mm/month) to April (140 mm/month) is reduced. The sunlight in July decreases to 6.0 hours and in August to 6.5 hours (Bezabih, 2021). Based on the composition of the fish species, the three Cyprinidae families (5 species, i.e., L. brevicephalus, L. megastoma, Labeobarbus nedgia, L. truttiformis and L. tsanensis), were reported so far at the dam site before the dam is built (Anteneh et al., 2013; Getahun et al., 2008). In addition, Cichlidae (O. niloticus) and Clariidae (C. gariepinus) were found on the Ribb River before and after the construction of the dam (Getahun et al., 2008).
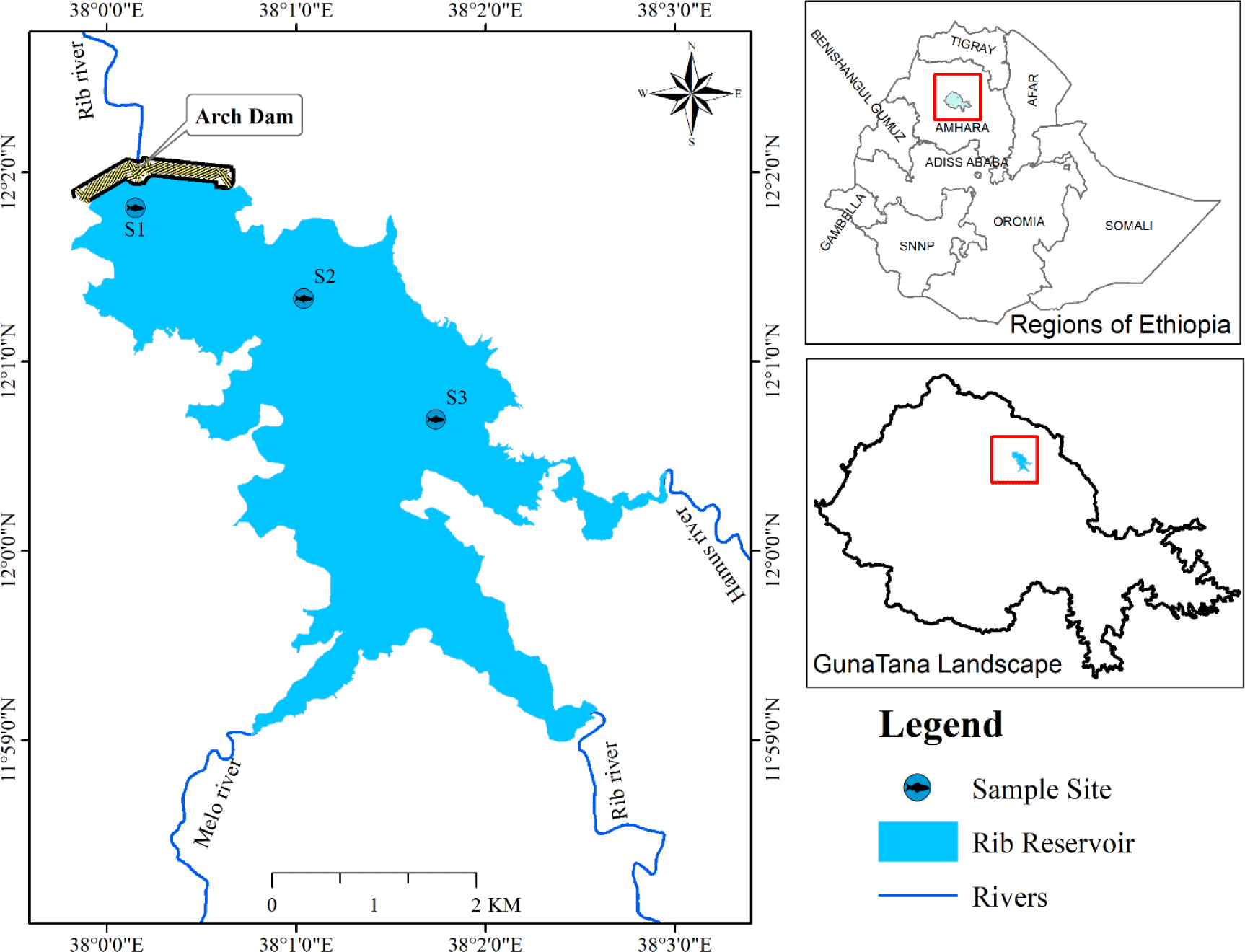
The study was conducted between February 2021 and October 2021. Between December and January 2020/21, the Fishery Research Team of the Guna Tana Integrated Field Research Development Centre at the University of Debre Tabor conducted a reconnaissance survey. These preliminary observations are important for understanding and identifying the various habitats and fishing activities of the river basin. Therefore, on the basis of preliminary processes, three sites have been selected considering the feasibility of establishing fishing nets, commercial fishing locations, depths of water, areas with vulnerability to fishing efforts, human effects, and nursery habitats. The sites were the S1 dam site (near the arch dam), S2 middle (middle of the reservoir), and the S3 inlet (entrance of Hamus River) indicated (Fig. 1).
Fish sample collection was carried out from February to October 2021. During this time, the monofilament gill net with 3.5 cm, 5, 6, 8, and 10 cm of stretched mesh and hook and a long line was used at three sampling sites at Ribb Reservoir. The nets were installed late at 3 o’clock and checked the next morning at 9 o’clock. The total length (TL, cm), fork length (FL, cm), standard length (SL, cm), and total weight (TW, g) of fish were measured using measuring boards with a sensitivity of 0.1 cm and 0.1 g by a sensitive balance. All fish specimens have been identified at the species level in the field.
The length-weight relationships among O. niloticus, C. gariepinus, and L. intermedius were calculated using a power function formula and the least square regression analysis was performed for both sexes (Tesch, 1978).
Where, W = weight, b = exponent; b = 3; implies isometric growth; when b < 3 means negative allometric growth and when b > 3 means positive allometric growth, and L = length of fish.
The condition factor of fishes in the reservoir was calculated using the equation below (Tesch, 1978).
Where K = condition factor, W = total body weight, and L = TL or fish FL.
The average length at 50% maturity (L50) was calculated using the length-frequency distribution of fishes with matured gonads (stage III–stage V). Finally, the L50 for both sexes was plotted by fitting a logistic regression model (Gunderson et al., 1980).
Here, Pm is the proportion of mature (%) at length (L) and a and b are fitted constants.
The calculation of fish eggs was performed considering ripe females (gonad stage IV). While gonad stage V was not considered because it is running. During the estimation of fecundity, the fishes were dissected and the ripe gonads were removed, measured to the nearest 0.01 g, and well maintained in 5% formalin solution in the field. Besides, those ripe ovaries were cut lengthways to permit the complete diffusion of the preservative at Debre Tabor University, Biology Department (Tesch, 1978). Accordingly, the preserved gonads were labeled and deposited in sampling jars for fecundity estimation. In laboratory experiments, the preserved ovarian cells were washed in tap water to remove ovarian sheaths by using a hand and sieve to protect flush out of the eggs. For the estimation of fecundity, a total count was used for the smallest gonads of O. niloticus. However, for the largest gonads, such as the C. gariepinus and L. intermedius species, the total number of eggs per ovaries was estimated by extrapolation by counting three sub-samples of 1 g of eggs per ovaries as indicated below.
The relationships of absolute fecundity (AF) to TL or FL, AF to the TW, and AF to gonad weight (GW) were assessed using the least-squares regression method.
Where AF = AF, X = represents the weight of the body, the TL, and the GW, a = the scale constant represents the intercept, and b = the allometric coefficient.
The data collected were interpreted and analyzed using version 20 of IBM SPSS statistical packages (IBM, Armonk, NY, USA). Descriptive statistics are used to present different data sets. The Chi-square test is used to test sex differences. One-way variation analysis (ANOVA) was used to observe differences in sex conditions factors. Finally, regression analysis is used to analyze and plan the relationship between fecundity with the total or FL, fecundity with total body weight, and, fecundity with GW of fish species in the reservoir.
Results
Distributions of the length-frequency of the three important commercial fishes in the reservoir were indicated in (Fig. 2). In this study, 752 Nile tilapia O. niloticus were caught. Of this catch, 353 were females whereas 399 were males. As for TL, females and males are 18.0–40.0 cm and males are 14.5–44.0 cm, respectively. The TW was 75.1 g to 1,150 g for female and 28.6 g to 1,450 g for male. The highest caught O. niloticus was found between 20.0–25.0 cm in the TL size class, which represented 67.02% of the total catch.
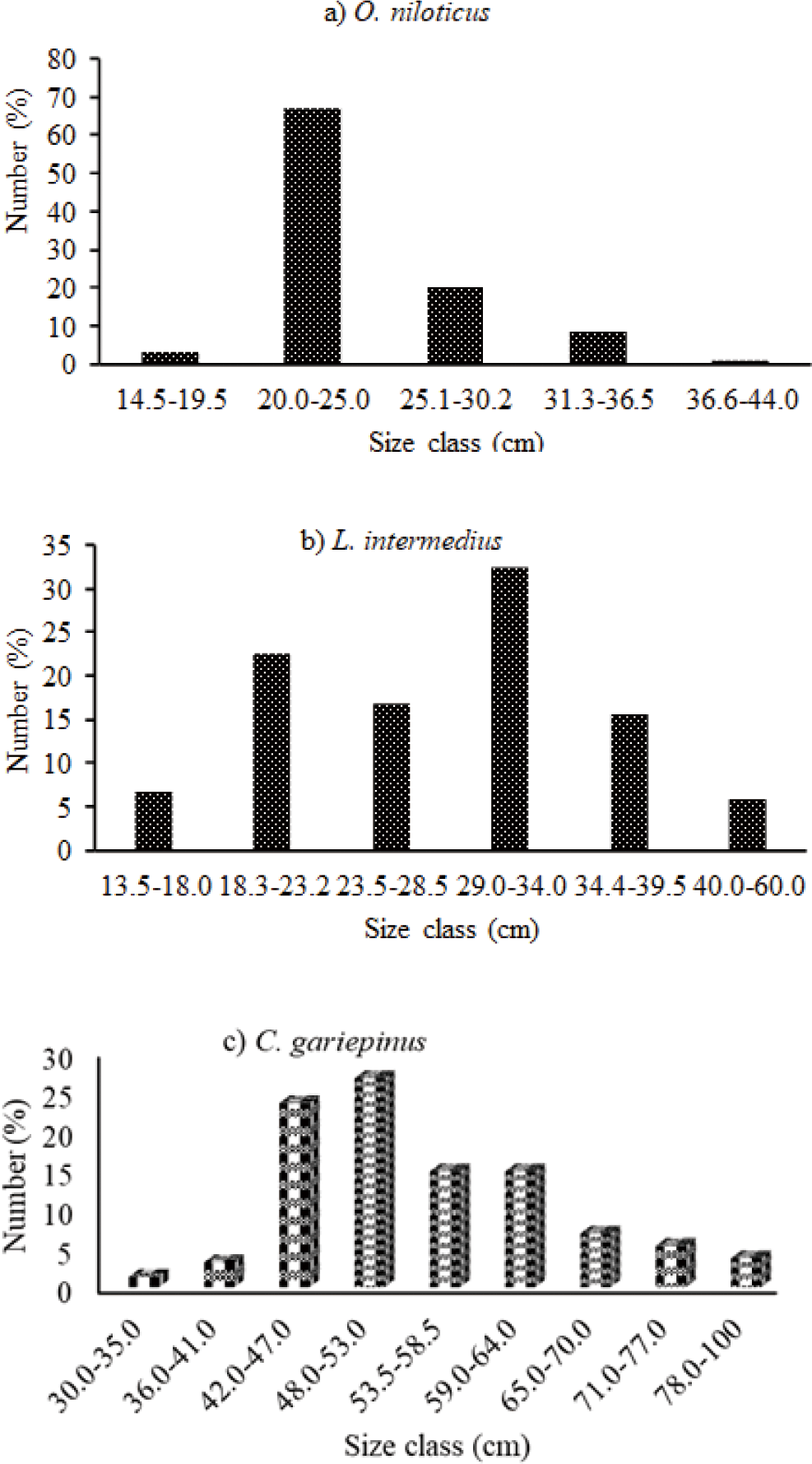
The overall 498 L. intermedius fish were collected. Their TL was found between 15.8 to 60.0 cm and 13.5 to 60.0 cm for females and males, respectively. The respective weight was found 29.4 to 1,200 g and 17.4 to 800 g for females and males, respectively. The 29.0–34 cm TL size class contributed the highest catch followed by the 18.3–23.2 cm TL size class. In this study, 379 C. gariepinus were collected. Accordingly, their TL was found between 31.0 to 85.0 cm TL and 30.0 to 100 cm TL and their body weight ranged from 98.0 to 4,100 g and 350 to 6,000 g for females and males, respectively. Among the size classes, 48.0–53.0 cm TL comprised the highest catch followed by the 42.0–47.0 cm TL size class.
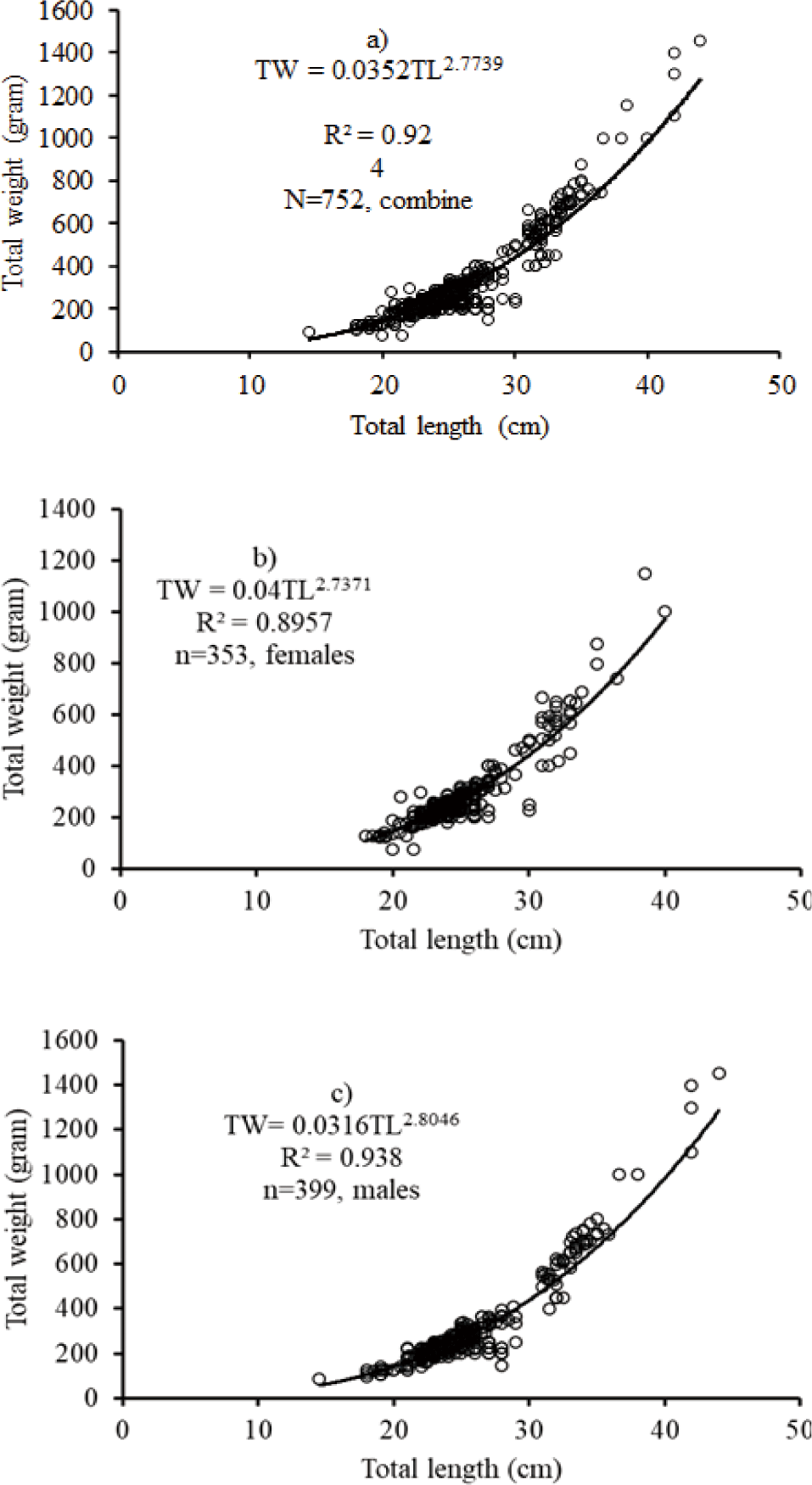
The relationships were curvilinear and exhibited a very significant (r2 > 0.8) of combined sex, female and male for O. niloticus, L. intermedius, and C. gariepinus, respectively (Figs. 3–5). The regression model for combined sex of O. niloticus was TW = 0.035TL2.77; r2 = 0.924, n = 752), for males TW = 0.0316TL2.8; r2 = 0.938, n = 399) and for females TW = 0.04TL2.74; r2 = 0.8957, n = 353). The results revealed that O. niloticus exhibited a negative allometric growth pattern in the reservoir. From the length-weight relationship data of O. niloticus, the fish grows in length rather than weight.
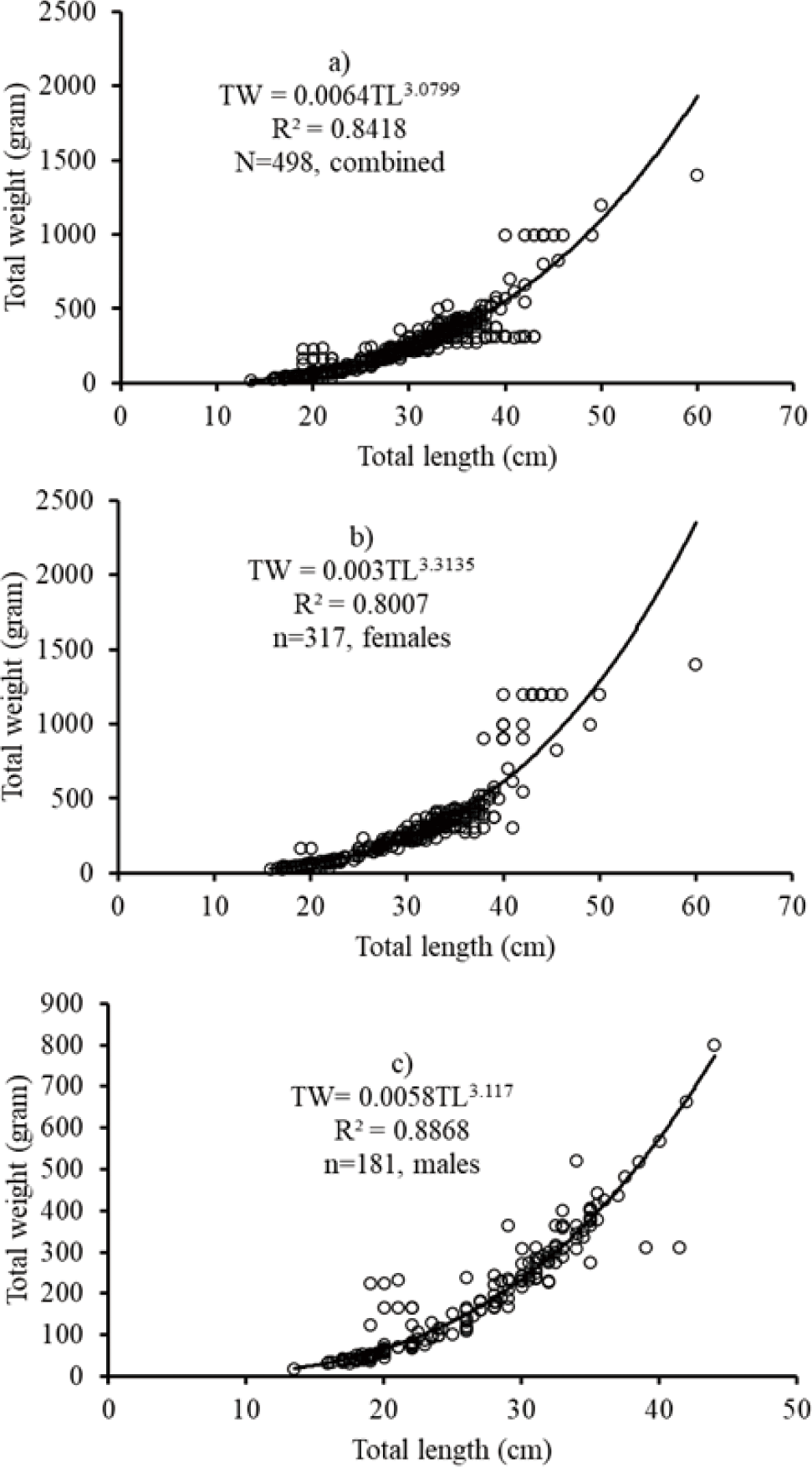
The length-weight relationship of the African big barb L. intermedius was curvilinear for the combined sexes (TW = 0.0064TL3.0799; r2 = 0.8418; n = 498). For males TW = 0.0058TL3.117; r2 = 0.887, n = 181) and females TW = 0.003TL3.3135; r2 = 0.8007, n = 317). The results revealed that isometric growth for combined sexes and males while positive allometric growth pattern for females.
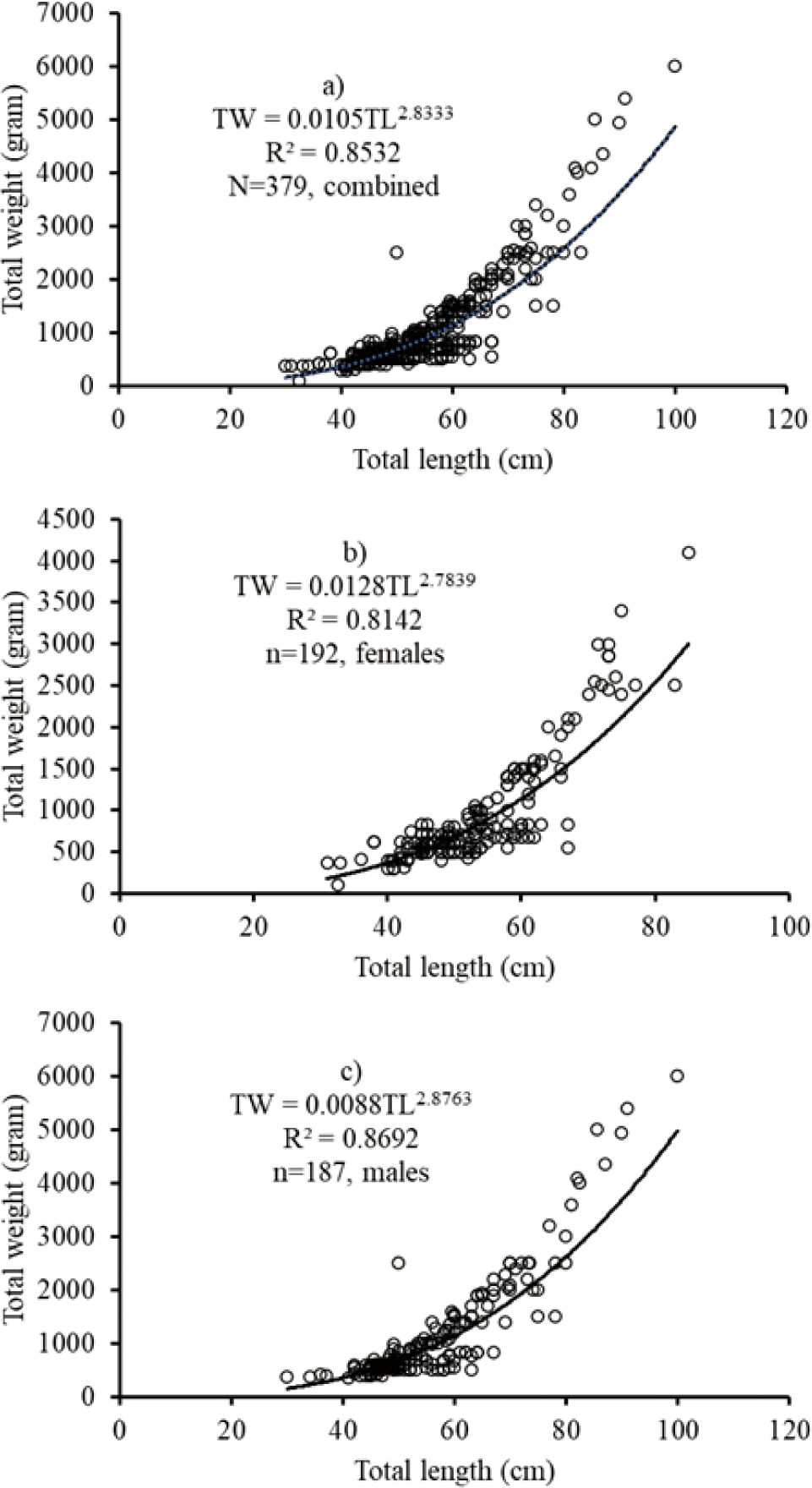
The data of the length-weight relationship showed that a negative allometric growth pattern was obtained in the mixed-sex African catfish C. gariepinus from the mixed-sex (TW = 0.0105TL2.833; r2 = 0.8532; n = 379). For males TW = 0.0088TL2.876; r2 = 0.8692, n = 187 and females TW = 0.0128TL2.784; r2 = 0.8142, n = 192. The results showed that a negative allometric growth pattern was obtained for combined sexes, males and females.
The average condition factors of three important commercial fish species were presented (Table 1). The mean condition factor for combined sexes of O. niloticus was 1.714 ± 0.009. In terms of separate sexes, the mean condition factors were 1.74 ± 0.01 and 1.69 ± 0.013 for females and males, respectively. The mean condition factor revealed a significant difference between the sexes of O. niloticus species (ANOVA, p < 0.05). The condition factor of L. intermedius was 0.85 ± 0.01 for mixed sexes. The condition factors 0.83 ± 0.01 and 0.88 ± 0.03 were belongs to females and males, respectively, and revealed a significant variation among sexes of L. intermedius (ANOVA, p < 0.05). The mean condition factor of the African catfish C. gariepinus was 0.563 ± 0.0085 for mixed sexes. In terms of sex-based condition factors, females had 0.56 ± 0.01 while males had 0.560 ± 0.013, which did not show significant variation between the sexes (ANOVA, p > 0.05). The mean condition factors of the three species exhibited a highly significant variation (ANOVA, p < 0.05). In this study, the Nile tilapia O. niloticus had better body condition than the African catfish C. gariepinus and the African big barb L. intermedius in Ribb Reservoir.
The sex ratio of three important commercial fish species was presented (Table 2). A total of 1,629 species of fish were collected. Out of these, 862 (52.9%) were females and 767 (47.1%) were males. The number of females and males species were 353 (40.95%), 317 (36.8%), 192 (22.3%), 399 (52.02%), 181 (23.6%), and 187 (24.4%) for O. niloticus, L. intermedius, and C. gariepinus, respectively. O. niloticus and C. gariepinus sex ratios showed no significant differences compared to the hypothetical 1:1 ratio (p > 0.05). However, L. intermedius showed that the sex ratio difference was significant (p < 0.05).
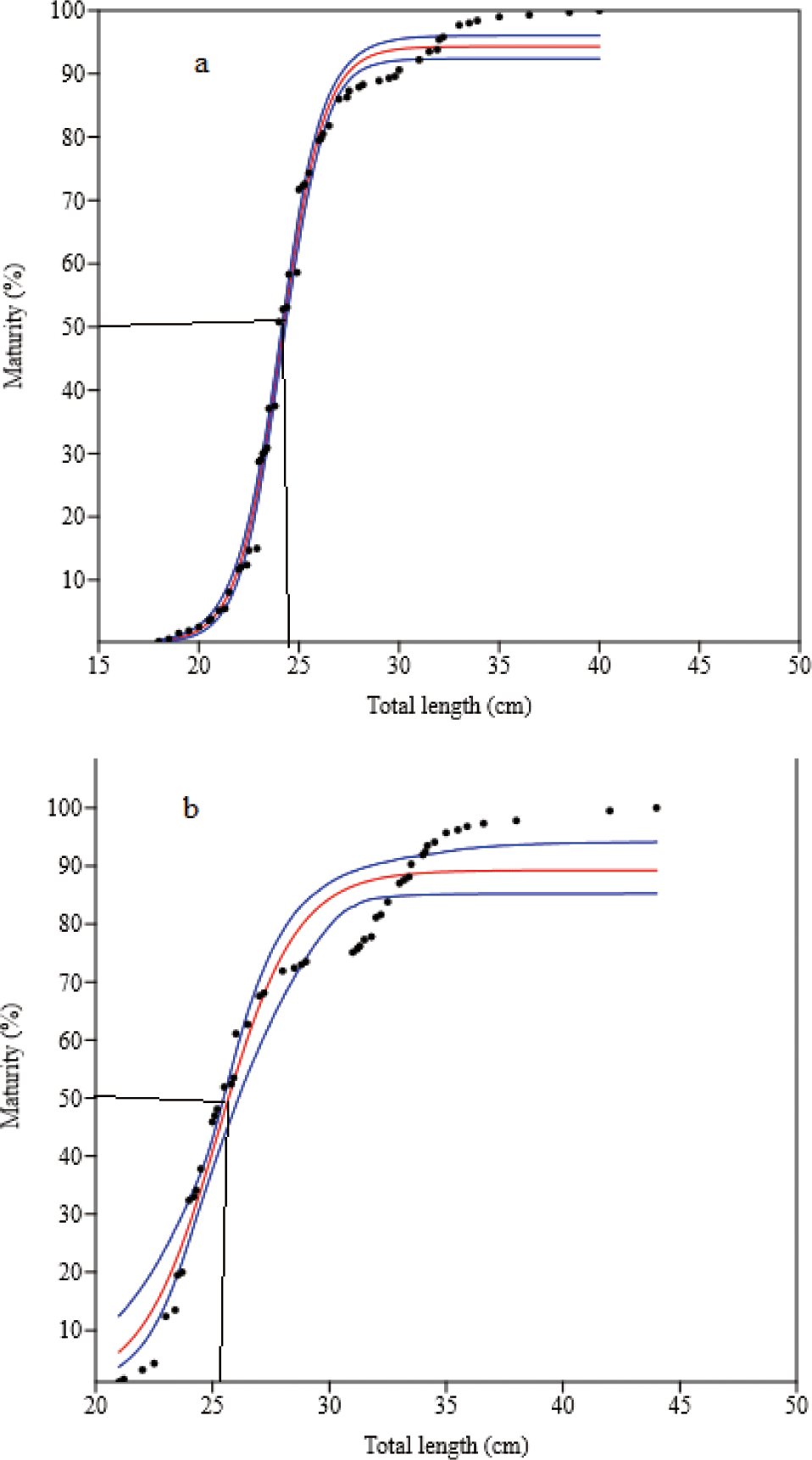
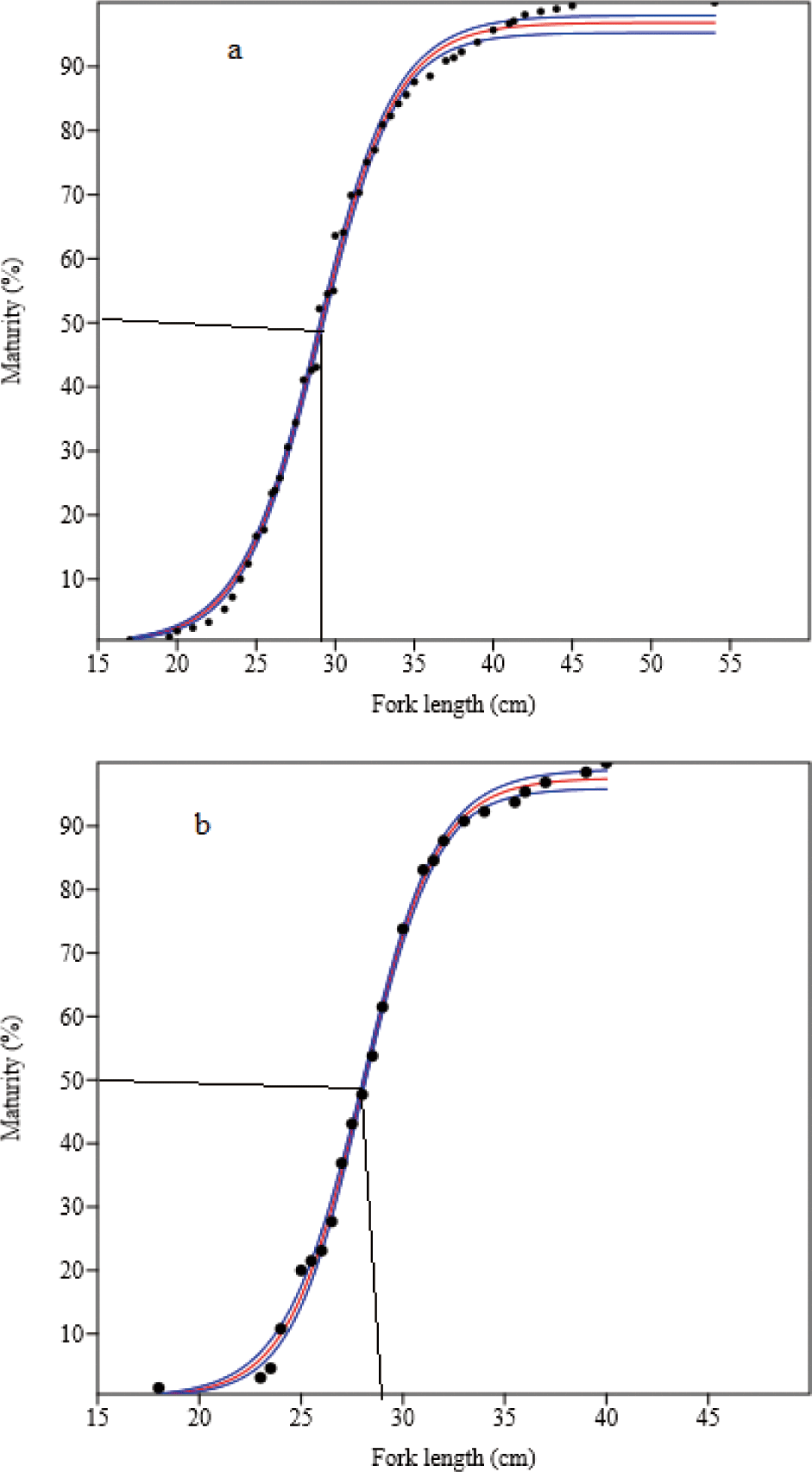
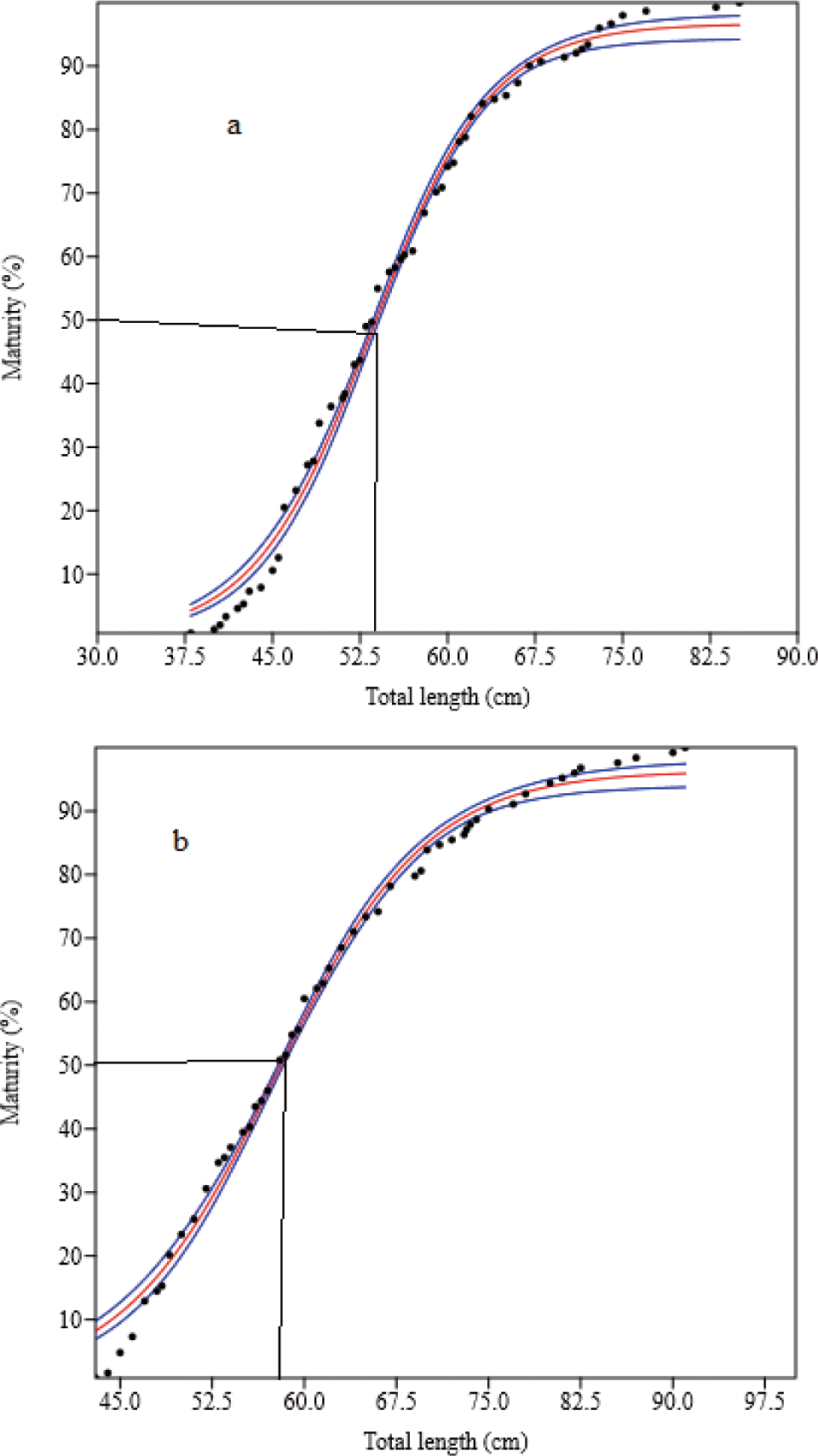
Four hundred and ninety-two mature (gonad stages III to V) O. niloticus were caught. Of these, 307 (61.9%) were females and 185 (37.6%) were males. The smallest sexually mature fish captured were 18.0 cm and 21.0 cm TL for females and males, respectively. Their body weights were 126.0 g and 182.0 g, respectively. L50O. niloticus was 24.0 cm TL for females and 25.4 cm TL males (Fig. 6). Females matured at a smaller size than males. Two hundred seventy-four matured (gonad stages III to V) L. intermedius were collected. Of the 209 fish caught (76.3%) were females and 65 (23.7%) were males. The smallest sexually matured fish caught were 17.0 cm and 18.0 cm FL for females and males, respectively and their weights were 48.4 g and 59.2 g, respectively. The L50 of the L. intermedius were 28.9 cm and 28.2 cm FL for females and males, respectively. Compared to females, males showed smaller size at first maturity than females (Fig. 7). Two hundred seventy-five matured (gonad stages III to V) C. gariepinus were collected. Of this sample, 151 (54.9%) were females and 124 (45.1%) males. The smallest sexually matured fish caught were 38.0 cm and 43.0 cm TL for females and males with body weights of 625 g and 510 g, respectively. The L50of the C. gariepinus were 53.2 cm and 57.5 cm TL for females and males, respectively. Relatively, females matured at a smaller size than males (Fig. 8).
The purpose for fecundity estimation, 139 ripe gonads (stage IV) females were selected. Of these ripe gonads, O. niloticus (73), C. gariepinus (38), and L. intermedius (28). Their length ranged from 20.5 to 35.0 cm TL, 46.0 to 83.0 cm TL, and 26.0 to 35.0 cm FL for O. niloticus, C. gariepinus and L. intermedius, respectively. Their body weights were also found between 150.5 to 876.4 g, 722.1 to 2,600 g, and 231.0 to 375.1 g, respectively. The GWs were found between 1.3 to 25.0 g, 10 to 450 g, and 8.6 to 24 g for O. niloticus, C. gariepinus, and L. intermedius, respectively. The number of eggs ranged from 100–947 eggs/g with AF 468–3,832 eggs, 200–1,000 eggs g with AF 2,752–136,420 eggs, and 505–900 eggs/g with AF 2,000–6,404 eggs for O. niloticus, C. gariepinus, and L. intermedius, respectively. The mean fecundities were 1,490 ± 91, 23,330 ± 5,070, and 3,096 ± 273 eggs for O. niloticus, C. gariepinus, and L. intermedius, respectively. The mean fecundity revealed a significant difference among the size classes and within the fish species (ANOVA, p < 0.05).
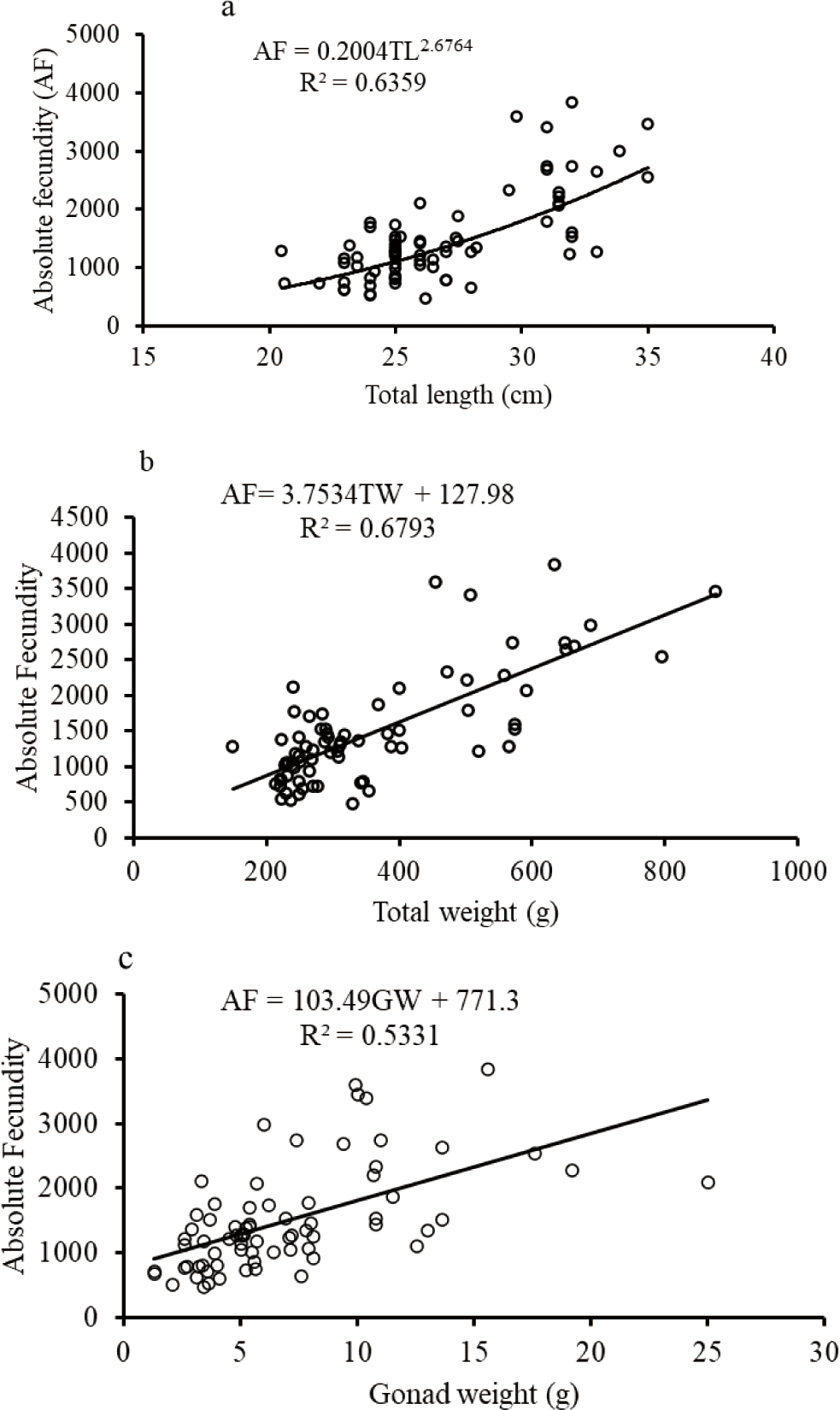
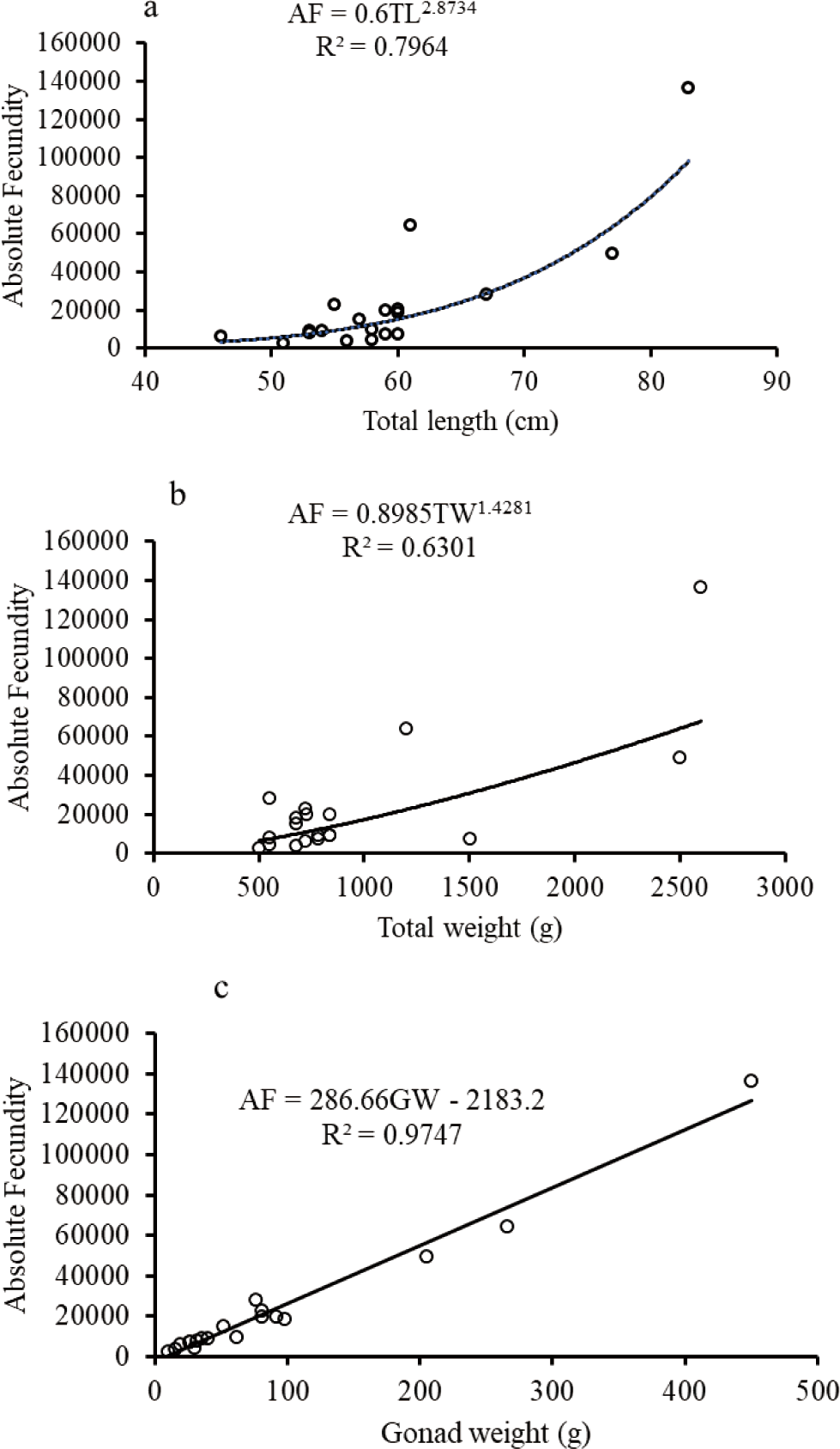
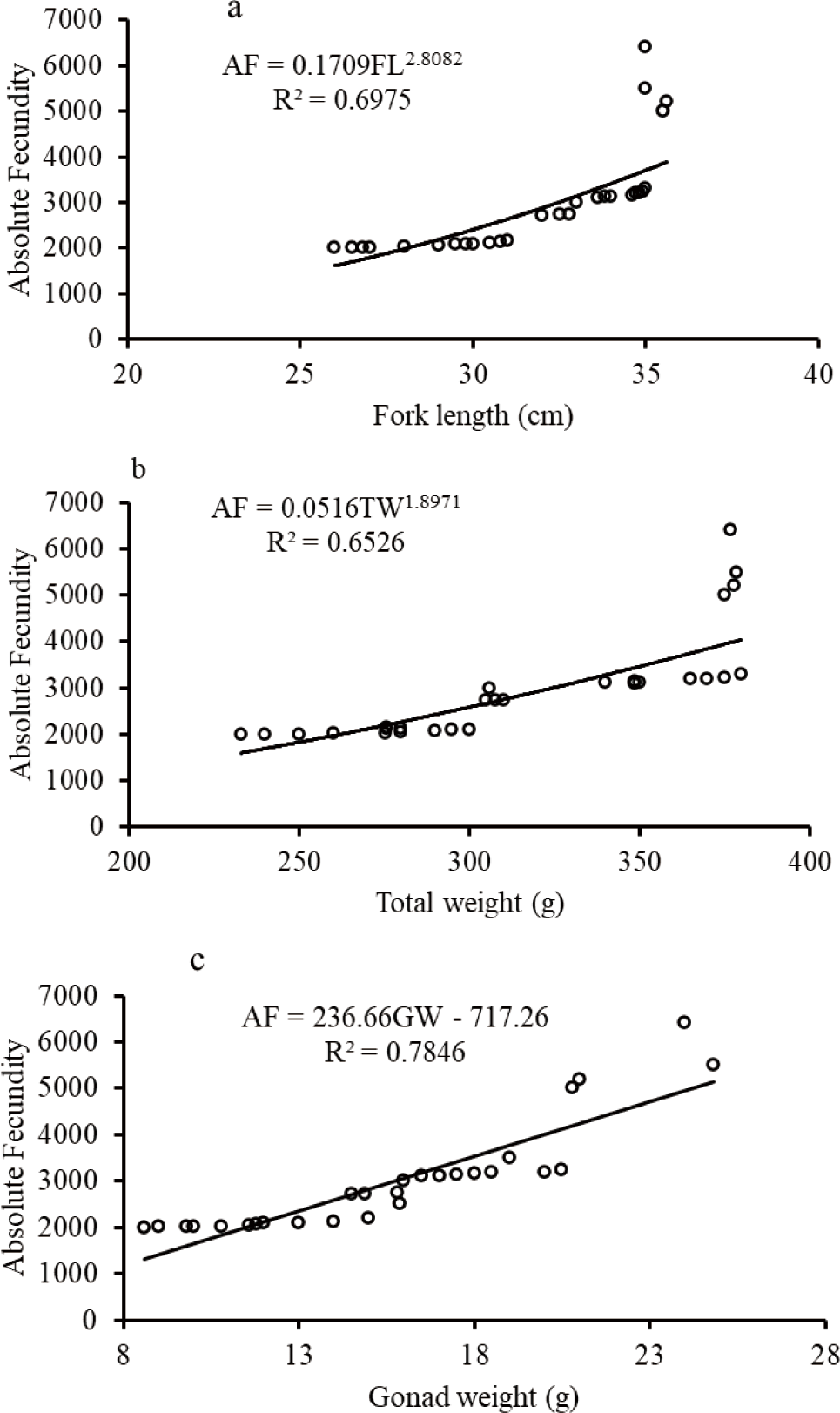
The biometric relationships such as fecundity-length and fecundity-body weight relationships except for O. niloticus were curvilinear while the GW-fecundity relationship was linear for O. niloticus, L. intermedius and C. gariepinus (Figs. 9–11). The AF was positively correlated with the TL, TW, and GW for O. niloticus, L. intermedius and C. gariepinus. The morphometric relationships showed significant relationships of AF with TL, TW, and GW for O. niloticus, L. intermedius and C. gariepinus (r2 > 0.6). The model with biometric data for TL, TW and GW were, AF = 0.2004 TL2.6764; r2 = 0.6359, AF = 3.7534 TW + 127.98; r2 = 0.6793, and (AF = 103.49 GW + 771.3; r2 = 0.5331), (AF = 0.6 TL2.8734; r2 = 0.7964; AF = 0.8985 TW1.4281; r2 = 0.6301 and AF = 286.66 GW–2,183.2; r2 = 0.9747), and (AF = 0.1709 FL2.8082; r2 = 0.6975, AF = 0.0516 TW1.8971; r2 = 0.66526, and AF = 236.66 GW–717.26; r2 = 0.7846) for O. niloticus, C. gariepinus and L. intermedius, respectively.
Discussion
In this study, O. niloticus had a maximum length of 44.0 cm TL. A similar size for the same species was also obtained from Lake Tana (Tefera et al., 2019). However, it was greater than the records reported in Tekeze Reservoir (38.5 cm) (Gebru, 2020), Lake Langeno (30.5) (Kebede et al., 2018), Lake Ziway (34 cm) (Abera et al., 2018), Koka Reservoir (35.2 cm) (Engdaw et al., 2013), Fincha Reservoir (42.0 cm) (Degefu et al., 2012), Amerti Reservoir (35.5 cm) (Hailu, 2014), Lake Babogaya (28.0 cm) (Hirpo, 2012), Lake Hayq (28.0 cm) (Worie & Getahun, 2014), and Lake Hawassa (39 cm) (Tesfaye et al., 2016). Conversely, it was smaller than the values reported in Lake Chamo (61 cm TL) (Teferi & Admassu, 2002), and Gilgel Gibe Reservoir (50 cm) (Wakjira, 2013) (Table 3).
| Species | Maximum length (cm, TL) | Water bodies | References |
|---|---|---|---|
| Oreochromis niloticus | 44.0 | Ribb Reservoir | Present study |
| 44.0 | Lake Tana | Tefera et al., 2019 | |
| 38.5 | Tekeze Reservoir | Gebru, 2020 | |
| 30.5 | Lake Langeno | Kebede et al., 2018 | |
| 34.0 | Lake Zeway | Abera et al., 2018 | |
| 35.2 | Lake Koka | Engdaw et al., 2013 | |
| 42.0 | Fincha Reservoir | Degefu et al., 2012 | |
| 35.5 | Amerti Reservoir | Hailu, 2014 | |
| 28.0 | Lake Babogaya | Hirpo, 2012 | |
| 28.0 | Lake Hayq | Worie & Getahun, 2014 | |
| 39.0 | Lake Hawassa | Tesfaye et al., 2016 | |
| 61.0 | Lake Chamo | Teferi & Admassu, 2002 | |
| 50.0 | Gilgel Gibe I Reservoir | Wakjira, 2013 | |
| Labeobarbus intermedius | 60.0 | Ribb Reservoir | Present study |
| 53.5 | Tekeze Reservoir | Gebru, 2020 | |
| 53.5 | Lake Langeno | Kebede et al., 2018 | |
| 43.8 | Lake Tana | Engdaw, 2014 | |
| 44.0 | Lake Zeway | Abera et al., 2018 | |
| 49.0 | Lake Koka | Dadebo et al., 2013 | |
| 54.0 | Gilgel Gibe I Reservoir | Wakjira, 2013 | |
| Clarias gariepinus | 100.0 | Ribb Reservoir | Present study |
| 68.5 | Lake Langeno | Kebede et al., 2018 | |
| 96.0 | Lake Zeway | Abera et al., 2018 | |
| 90.0 | Lake Chamo | Dadebo et al., 2011 | |
| 102.0 | Lake Babogaya | Abera et al., 2014 |
In this study, the maximum TL for L. intermedius was 60.0 cm. However, the value obtained was greater than the value recorded in Tekeze Reservoir (53.5 cm TL) (Gebru, 2020), Lake Langeno (53.5 cm TL) (Kebede et al., 2018), Lake Tana (43.8 cm TL) (Engdaw, 2014), and Lake Ziway (44 cm) (Abera et al., 2018), Lake Koka (49.0 cm TL) (Dadebo et al., 2013) and Gilgel Gibe I Reservoir (54.0 cm TL) (Wakjira, 2013) (Table 3).
The maximum length noted for C. gariepinus was 100.0 cm TL. It was greater than the values reported from Lake Langeno (68.5 cm) (Kebede et al., 2018), Lake Zeway (96.0 cm) (Abera et al., 2018), and Lake Chamo (90.0 cm) (Dadebo et al., 2011). However, it was slightly smaller than previous findings reported in Lake Babogaya (102.0 cm) (Abera et al., 2014) (Table 3).
In different water bodies, the difference in fish size of similar species could probably be attributed to the variability of food access, anthropogenic pressures, fishing pressure, the type of fishing gear used, the availability of predators that prey on the smallest fish, and environmental variable of the water quality. The interaction of biological, physical, chemical and overfishing factors in the water could govern the sizes of fish species. In this study, the maximum sizes of fish were found in a good way.
The TL of the biometric relationship with the TW of O. niloticus was curvilinear and showed a negative allometric growth pattern (b < 3). A negative allometric growth pattern refers to the fish growing in length than weight, while positive allometric growth is defined as the fish growing in weight than length. The isometric growth pattern refers to body weight growing at a proportional rate as the size of the fish increases. Several investigators have documented allometric growth patterns of O. niloticus in different water bodies of Ethiopia Lake Tana (Tewabe, 2014), Lake Chamo (Teferi & Admassu, 2002), Koka Reservoir (Engdaw et al., 2013), Gilgel Gibe Reservoir (Wakjira, 2013) and Lake Langeno (Kebede et al., 2018).
In this study, the recorded regression slope value (b = 3) from the length-weight relationship data of L. intermedius has obeyed the cube law (Tesch, 1978). The value of the exponent coefficient indicated the isometric growth pattern (b = 3), indicating that the length and weight of the fish grow at almost the same rate. Comparable findings were made at Tekeze Reservoir (Gebru, 2020), some tributaries of Lake Tana (Teshome et al., 2015), Lake Tana (Engdaw, 2014), Blue Nile River (Awoke et al., 2015), Borkena and Mille Rivers (Tessema et al., 2012), and Ribb River (before the dam construction) (Getahun et al., 2008). A negative allometric growth patterns (b < 3) were also reported in Lake Langeno (Kebede et al., 2018), and Gilgel Gibe Reservoir (Wakjira, 2013).
A negative allometric growth pattern (b < 3) was recorded for C. gariepinus. Similar findings were also reported in Lake Lugo South Wollo (Mekonnen et al., 2019), Lake Langeno (Kebede et al., 2018), Lake Babogaya (Abera et al., 2014), Lake Zeway (Abera et al., 2018), and Lake Tana (Tewabe, 2014). Human pressures, such as irrigation practices and water abstraction around the watershed, lead to the alteration of environmental water quality variables. These could affect the availability of prey items in the reservoir, and this might hinder the fish growth pattern. The evidence is poor land use practices in the watershed of the Ribb basin, particularly in the reservoir (personal observation and communication). Therefore, the management of the watershed area is required to make suitable habitats for the harbored fish.
In this finding, the mean condition factor for O. niloticus was 1.71. It was the smallest of the findings conducted in Lake Langeno (1.74) (Kebede et al., 2018), in the same lake (1.84) (Tesfaye & Tadesse, 2008), Lake Hayq (1.83) (Tessema et al., 2019), Lake Zeway (1.82) (Abera et al., 2018), Lake Tana (1.89) (Tewabe, 2014), Lake Babogaya (2.13) (Hirpo, 2012) and Fincha Reservoir (1.83) (Degefu et al., 2012). However, it was greater than the findings conducted at Tekeze Reservoir (1.43) (Gebru, 2020) (Table 4). The result showed approximately good body condition of this species as compared to the earlier reports.
| Species | Condition factors | Water bodies | References |
|---|---|---|---|
| Oreochromis niloticus | 1.71 ± 0.5 | Ribb Reservoir | Present study |
| 1.74 ± 0.4 | Lake Langeno | Kebede et al., 2018 | |
| 1.84 ± 0.34 | Lake Langeno | Tesfaye & Tadesse, 2008 | |
| 1.83 ± 0.35 | Lake Hayq | Tessema et al., 2019 | |
| 1.82 ± 0.5 | Lake Zeway | Abera et al., 2018 | |
| 1.89 ± 0.6 | Lake Tana | Tewabe, 2014 | |
| 2.13 ± 0.8 | Lake Babogaya | Hirpo, 2012 | |
| 1.83 ± 0.4 | Fincha Reservoir | Degefu et al., 2012 | |
| 1.43 ± 0.3 | Tekeze Reservoir | Gebru, 2020 | |
| Labeobarbus intermedius | 0.85 ± 0.23 | Ribb Reservoir | Present study |
| 1.88 ± 0.6 | Tekeze Reservoir | Gebru, 2020 | |
| 1.33 ± 0.4 | Lake Langeno | Kebede et al., 2018 | |
| 1.29 ± 0.2 | Lake Tana | Gebremedhin et al., 2014 | |
| 1.21 ± 0.5 | Tributaries of Lake Tana | Teshome et al., 2015 | |
| 1.73 ± 0.6 | Lake Zeway | Abera et al., 2018 | |
| Clarias gariepinus | 0.56 ± 0.3 | Ribb Reservoir | Present study |
| 0.58 ± 0.23 | Lake Langeno | Kebede et al., 2018 | |
| 0.64 ± 0.3 | Lake Babogaya | Abera et al., 2014 | |
| 0.76 ± 0.32 | Lake Zeway | Abera et al., 2018 |
The mean body condition factor for L. intermedius was 0.85. It was by far the smallest value as compared to the reports made from Tekeze Reservoir (1.88) (Gebru, 2020), Lake Langeno (1.33) (Kebede et al., 2018), Lake Tana (1.29) (Gebremedhin et al., 2014), and some tributary rivers of Lake Tana (1.21) (Teshome et al., 2015), and Lake Zeway (1.73) (Abera et al., 2018) (Table 4). The result revealed that this species of fish exhibited poor body condition compared to previous reports in different water bodies in Ethiopia.
The mean body condition factor for C. gariepinus was 0.56. It is almost similar to Lake Langeno (0.58) (Kebede et al., 2018). However, it was less than in Lake Babogaya (0.64) (Abera et al., 2014), and Lake Zeway (0.76) (Abera et al., 2018) (Table 4). The body condition factor reflects the well-being of fish in water bodies. It is affected by variations in food availability, water level, water quality, and water temperature that governs the body condition of fishes (Gebru, 2020). The larger mean value of the body condition factor describes the higher energy content, high food availability, reproductive potential, and favorable environmental conditions for fish species (Kebede et al., 2018). Tessema et al. (2012) documented that the poor body condition of fish species is associated with the low availability of food items such as insects, snails, and other invertebrates when missed in water bodies. Based on the obtained values of the condition factor, O. niloticus was by far the most well-adapted to the environmental conditions in the reservoir than other species of fish.
In the present catch, the females were more dominant than the males among the three species and significantly different from the 1:1 ratio (Table 2). The females of C. gariepinus and L. intermedius were dominated in the catch, while the males dominated more than the females in the case of O. niloticus. Differences in proportions of sex ratios were documented in some water bodies of Ethiopia in the Tekeze Reservoir (Gebru, 2020), Lake Langeno (Kebede et al., 2018), Lake Babogaya (Hirpo, 2012), Lake Beseka (Hirpo, 2013), Lake Hayq (Tessema et al., 2019), Lake Tana (Tewabe, 2014). The probable reasons for the different sex ratio proportions within females and males might be due to the behavioral (breeding or other) differences among sexes or species, this might make one sex to be more exposed to the fishing gear, and ecology of the fishes also causes the deviation of sex ratio (Teferi & Admassu, 2002).
L50 refers to the smallest size reached at maturity of the 50% population. As a result, fish catch after reaching a L50 is important for sustainable fisheries. It is recommended that the size at harvest would exceed the size at first maturity (Tefera et al., 2019) to sustain the fish stock. Length at first harvest and L50 can determine the sustainability of fish resources in water bodies.
In this study, females of O. niloticus matured at a smaller size than males. Comparable studies were conducted in different water bodies of Ethiopia in Lake Tana (Tewabe, 2014), Lake Hawassa (Tesfaye et al., 2016), and Tekeze Reservoir (Gebru, 2020). However, studies made in Lake Tana (Tefera et al., 2019), and Lake Langeno (Kebede et al., 2018) showed maturation at a smaller size of males rather than females.
The L50 for O. niloticus were 24.0 cm and 25.4 cm TL for females and males, respectively. A comparable study was conducted in Tekeze Reservoir 24.2 cm and 24.8 cm TL (Gebru, 2020) for females and males, respectively. In contrast, the L50 values were greater than in Lake Tana (19.6 cm and 20.5 cm TL) (Tefera et al., 2019), Lake Langeno (17.4 cm and 17.2 cm TL) (Kebede et al., 2018), in Lake Langeno (19.5 cm TL for both sexes) (Tesfaye & Tadesse, 2008), Lake Hawassa (18.8 cm and 19.8 cm TL) (Tesfaye et al., 2016), Fincha Reservoir (21.2 cm and 23.4 cm TL) (Degefu et al., 2012), Lake Beseka (14.0 cm and 16.0 cm TL) (Hirpo, 2013) and Lake Hayq (14.5 cm and 15.5 cm TL) (Worie & Getahun, 2014) for females and males, respectively. The L50 was less than when compared to the findings conducted from Lake Chamo (42.0 cm TL for both sexes) (Teferi et al., 2000), and Koka Reservoir (30.8 cm and 34.6 cm TL) (Tesfaye et al., 2016) for females and males, respectively (Table 5).
| Species | Size at maturity (L50, cm TL) | Water bodies | References | |
|---|---|---|---|---|
| Females | Males | |||
| Oreochromis niloticus | 24.0 | 25.4 | Ribb Reservoir | Present study |
| 24.2 | 24.8 | Tekeze Reservoir | Gebru, 2020 | |
| 19.6 | 20.5 | Lake Tana | Tefera et al., 2019 | |
| 17.4 | 17.2 | Lake Langeno | Kebede et al., 2018 | |
| 19.5 | 19.5 | Lake Langeno | Tesfaye & Tadesse, 2008 | |
| 18.8 | 19.8 | Lake Hawassa | Tesfaye et al., 2016 | |
| 21.2 | 23.4 | Fincha Reservoir | Degefu et al., 2012 | |
| 14.0 | 16.0 | Lake Beseka | Hirpo, 2013 | |
| 14.5 | 15.5 | Lake Hayq | Worie & Getahun, 2014 | |
| 42.0 | 42.0 | Lake Chamo | Teferi et al., 2000 | |
| 30.8 | 34.6 | Koka Reservoir | Tesfaye et al., 2016 | |
| Clarias gariepinus | 53.2 | 57.5 | Ribb Reservoir | Present study |
| 52.0 | 58.0 | Lake Chamo | Dadebo et al., 2011 | |
| 31.5 | 32.5 | Lake Langeno | Kebede et al., 2018 | |
| 28.7 | 27.0 | Lake Zeway | Abera et al., 2018 | |
| 50.0 | 56.0 | Lake Babogaya | Abera et al., 2014 | |
| 57.7 | 43.2 | Lake Tana | Tewabe, 2014 | |
| Species | Size at maturity (L50, cm FL) | Water bodies | References | |
|---|---|---|---|---|
| Females | Males | |||
| Labeobarbus intermedius | 28.9 | 28.2 | Ribb Reservoir | Present study |
| 20.84 | 22.05 | Tekeze Reservoir | Gebru, 2020 | |
| 22.6 | 22.6 | Dirma and Megech tributary rivers | Anteneh et al., 2007 | |
| 26.0 | 26.0 | Tekeze and Blue Nile basins | Tewabe et al., 2009 | |
| 30.5 | 29.5 | Lake Langeno | Kebede et al., 2018 | |
| 32.7 | 25.9 | Lake Tana | Tewabe, 2014 | |
The L50 values for L. intermedius were 28.9 cm and 28.2 cm FL for females and males, respectively. The males showed somewhat maturation at a smaller size than the females. The L50 was greater than in Tekeze Reservoir 20.84 cm and 22.05 cm SL (Gebru, 2020), Dirma and Megech tributary rivers 22.6 cm FL for both sexes (Anteneh et al., 2007), and some rivers of the Tekeze and Blue Nile basins 26.0 cm FL for both sexes (Tewabe et al., 2009) for females and males, respectively. However, it was less than the studies conducted in Lake Langeno of 30.5 cm and 29.5 cm FL (Kebede et al., 2018), and Lake Tana of 32.7 cm and 25.9 cm FL (Tewabe, 2014) for females and males, respectively (Table 5). Furthermore, the findings confirmed the early maturity of the males, in line with the present study.
C. gariepinus was attained at 53.2 cm and 57.5 cm TL L50 for females and males, respectively. Consequently, females matured at a smaller size than males. A comparable study was conducted in Lake Chamo 52.0 cm and 58.0 cm TL for females and males (Dadebo et al., 2011). However, it was greater than the reports in Lake Langeno (31.5 cm and 32.5 cm TL) (Kebede et al., 2018), Lake Zeway (28.7 cm and 27.0 cm TL) (Abera et al., 2018), and Lake Babogaya (50.0 cm and 56.0 cm TL) (Abera et al., 2014) for females and males, respectively (Table 5). A study made in Lake Tana (57.7 cm and 43.2 cm TL for females and males) (Tewabe, 2014) showed the difference in L50 between the sexes. The maturity at a smaller size of fish species reflects those fishes living in a stressful environment, under fishing pressure, and the less availability of food items (Kebede et al., 2018). For example, fish in poor conditions mature to a shorter length than fish in better conditions (Tirunesh, 2015). Furthermore, in this study, the length-frequency distribution of fish species could be affected because fish migrate through the outlet when water is needed for irrigation purposes and the reservoir is full to release excess water. As a result, the smallest fishes preyed using birds at the outlet site (personal observation). This could affect the size at maturity of fishes in the reservoir.
The number of eggs obtained for O. niloticus was found between (468–3,832 eggs/ovary) with a mean of 1,490 ± 91 eggs/ovary. The fecundity (eggs/ovary) of this study was larger than in studies conducted in the Tekeze Reservoir (272 to 3,337) (Gebru, 2020), Lake Langeno (187 to 978) (Kebede et al., 2018), Lake Hawassa (304 to 967) (Dadebo et al., 2011), Lake Tana (495 to 1,243) (Tewabe, 2014), Lake Hayq (290 to 1,287) (Worie & Getahun, 2014). However, it was less than the fecundity (eggs/ovary) of the same species and in Lake Chamo (1,047 to 4,590) (Teferi et al., 2001) (Table 6).
| Species | Eggs per ovary | Water bodies | References |
|---|---|---|---|
| Oreochromis niloticus | 468–3,832 | Ribb Reservoir | Present study |
| 272–3,337 | Tekeze Reservoir | Gebru, 2020 | |
| 187–978 | Lake Langeno | Kebede et al., 2018 | |
| 304–967 | Lake Hawassa | Dadebo et al., 2011 | |
| 495–1,243 | Lake Tana | Tewabe, 2014 | |
| 290–1,287 | Lake Hayq | Worie & Getahun, 2014 | |
| 1,047–4,590 | Lake Chamo | Teferi et al., 2001 | |
| Labeobarbus intermedius | 2,000–4,604 | Ribb Reservoir | Present study |
| 1,078–6,532 | Lake Langeno | Kebede et al., 2018 | |
| 1,345–7,235 | Blue Nile River | Awoke et al., 2015 | |
| 1,761–8,367 | Tributaries of Lake Tana | Anteneh et al., 2007 | |
| 1,457–10,658 | Tekeze Reservoir | Gebru, 2020 | |
| 1,935–11,224 | Lake Tana | Gebremedhin et al., 2014 | |
| 1,265–13,289 | Gelda and Gumara Rivers | Teshome et al., 2015 | |
| Clarias gariepinus | 2,752–136,420 | Ribb Reservoir | Present study |
| 22,600–211,442 | Lake Langeno | Kebede et al., 2018 | |
| 11,000–580,571 | Lake Babogaya | Admassu & AberaL, 2015 | |
| 5,000–1,240,000 | Lake Chamo | Dadebo et al., 2011 |
In this study, the fecundity of C. gariepinus was (2,752–136,420 eggs/ovary) with a mean of 23,330 ± 5,070 eggs. This is very small compared to other water bodies for the same fish species in Lake Langeno (22,600 to 211,442 eggs/ovary with a mean of 141,466 eggs) (Kebede et al., 2018), Lake Babogaya (11,000 to 580,571 eggs/ovary) (Admassu & AberaL, 2015), and Lake Zeway (10,000 to 560,000 eggs/ovary) (Abera et al., 2014) and Lake Chamo (5,000 to 1,240,000 eggs/ovary) (Dadebo et al., 2011) (Table 6).
The fecundity recorded for L. intermedius of this study was (2,000–6,404 eggs/ovary) with a mean of 3,096 ± 273 eggs, and this result is comparable to some other studies conducted in Lake Langeno (1,078 to 6,532 eggs/ovary with the mean fecundity of 3,055 eggs/ovary) (Kebede et al., 2018), Blue Nile River (1,345 to 7,235 eggs/ovary) (Awoke et al., 2015), and some tributaries of Lake Tana (1,761 to 8,367 eggs/ovary) (Anteneh et al., 2007). But it was smaller than reported in Tekeze Reservoir (1,457 to 10,658 eggs/ovary with a mean of 4,788 eggs) (Gebru, 2020), Lake Tana (1,935 to 11,224 eggs/ovary) (Gebremedhin et al., 2014), and Gelda and Gumara Rivers (1,265 to 13,289 eggs/ovary) (Teshome et al., 2015). In this study, the fecundity (eggs/ovary) (mean of 1,490, 23,330, and 3,096) for O. niloticus, C. gariepinus, and L. intermedius respectively (Table 6). These obtained fecundities were low among the fish species particularly, the fecundity recorded for C. gariepinus was by far very low. According to the current result, Ogunola et al. (2018) stated that fecundity is species-specific, linked with genetic features, size, age, environmental aspects, and physiological circumstances of the fish. For example, large female fish in better condition tend to have higher fertility than fish in poor body condition. Furthermore, fertility and associated factors such as age, growth rate, size, genetic composition, adequate food supply, and environmental factors such as water temperature were documented by (Adams et al., 2015) a study conducted in Lake Eleiyele, Ibadan, Nigeria. For example, most of the fish show a gradual increase in fecundity with their age, while the fish become old, and the eggs to be produced start to decline (Adams et al., 2015). The water temperature also affects the reproductive biology of fishes by interfering with the spawning periodicity and recruitment achievement as well. Consequently, under unfavorable water temperatures, adult female fishes are exposed to balance their energy for survival and reproductive capability, which leads to less fecundity (Ogunola et al., 2018). The reason for the low fertility in this study might also be linked to fluctuations in water level and contaminants. Unexpected changes in water levels could also cause a decline in fecundity fish. For example, the study area experienced different water levels in the seasons of the year. Similarly, the reproductive performance of fishes is also negatively affected via various environmental pollutants (caused by anthropogenic pressures) such as heavy metals, pesticides, and polycyclic aromatic hydrocarbons. Furthermore, water pollutants, agricultural waste, pesticides, and trace metals could have histopathological negative effects on the reproductive tissues of fish gonads, thus decreasing the ability of the fish to reproduce. In a polluted reservoir, low fecundity was obtained between fish species. Fecundity is also related to the availability of quality food resources. The low or high fecundity of fishes could reflect the abundance of food and water quality parameters in the waters. Scarcity of food in water bodies can cause low fecundity. In conclusion, low fertility, in this study, could be traced to the fluctuation of water levels, variation of physicochemical water quality parameters, scarcity of quality food, and anthropogenic pressures in Ribb Reservoir.
In the current finding, the relationships of the fecundity with total or FL and fecundity with TWs of fishes except for O. niloticus were curvilinear while the relationship of fecundity with GW was linear. Several studies were conducted to confirm the association of biometric relationships (fecundity-TL or FL, fecundity body weight, and fecundity GW) (Awoke et al., 2015; Gebremedhin et al., 2014; Gebru, 2020; Kebede et al., 2018; Teferi et al., 2001).
Conclusion
Among the length-frequency distributions, the maximum sizes were 44 cm, 60 cm, and 100 cm TL for O. niloticus, L. intermedius, and C. gariepinus, respectively. The length-weight relationships were curvilinear and showed a negative allometric growth pattern except for L. intermedius (isometric). O. niloticus was found to have better body condition than L. intermedius and C. gariepinus. Female fishes were dominated in the total catch except for O. niloticus where males dominated. Except for L. intermedius females exhibited early maturation than males. The fecundity for C. gariepinus was very low. The fecundity length and fecundity-body weight relationships except (O. niloticus) were curvilinear, while the GW-fecundity relationship was linear for O. niloticus, C. gariepinus and L. intermedius. In conclusion, the responsible limiting factors for biometric relationships and reproductive biology fish could be a shortage of quality foods, fluctuation of environmental variables, variations in water level, agriculture waste discharges and anthropogenic pressures (expansion of irrigation practices around the watershed) thus, appropriate management and community awareness are required to sustain fisheries in the reservoir.