Introduction
Seaweed cultivation in Indonesia has become a significant contributor to economic growth, which can be attributed to several factors. First, seaweed cultivation encourages investment. Seaweed is known for its versatile applications, positioning seaweed derivatives as commodities closely integrated into the production chains of various industries, driving ongoing investment in the sector. Second, seaweed cultivation generates extensive employment opportunities. Rafael et al. (2015) revealed that global seaweed production has increased exponentially over the past half century in around 50 countries in the world, so that with increasing production, it is the impact of increasing human resource absorption in seaweed farming. Jobs are created not only in seaweed cultivation but also in the post-harvest seaweed processing industry. Third, seaweed cultivation is a significant source of income (Langford et al., 2022). Although Indonesian seaweed cultivation is characterized by simple technology and low capital (Neish, 2013), this commodity is able to generate income from exports and from the gross domestic product (GDP) of the aquaculture and fisheries sector. Indonesian seaweed production has shown tremendous growth over the past two decades and has contributed significantly to global production (Langford et al., 2024).While seaweed was first cultivated for commercial gain in Indonesia in the mid-1980s in Bali, the center of seaweed cultivation is now concentrated in the Sulawesi region, especially South Sulawesi, with a notable increase in national production from 26.4% (2.42 million tons) in 2013 to 34.7% (3.66 million tons) in 2017 (Larson et al., 2021). The dominant seaweed cultivated is the red alga Kappaphycus alvarezii, with the trade name cottonii (Moreno et al., 2021). However, the widespread practice of intensive seaweed cultivation throughout the year in recent years has resulted in a decline in nutrient levels in the waters that support seaweed farming. In South Sulawesi’s coastal waters, the concentration levels of nutrients crucial for seaweed nutrition, including nitrate (NO3–) and phosphate, increasingly fail to meet seaweed farming standards. Studies have recorded NO3– concentrations of 0.1–0.5 mg/L in Bantaeng Regency and 0.22 mg/L in Takalar Regency (standard: 0.9–3.5 mg/L), with phosphate concentrations of 0.35–0.5 mg/L in Bantaeng Regency and 0.08 mg/L in Takalar Regency (standard: 0.1–0.5 mg/L) (Asni, 2015; Ramdhan et al., 2018).
Such nutrient deficiency increases the vulnerability of seaweed to bacterial disease, often referred to as “ice-ice” disease and is a common disease in Kappaphycus farming throughout Indonesia. This disease is a major factor causing poor seaweed quality, and is characterized by a change in seaweed color, from the normal (typically reddish brown) color to an (often translucent) whitish hue (Capacio et al., 2024). Similarly, in Japan, nutrient depletion has been linked to color changes in nori seaweed, attributed to pigment reduction (Kittiwanich et al., 2016; Nishikawa et al., 2009). Since the enactment of restrictions on the disposal of organic waste into waters by the Japanese central government in the 1980s, coastal waters in Japan have become oligotrophic (Yamamoto et al., 2021). While Japan addressed this challenge with relaxed organic waste disposal regulations (Tomita et al., 2015), Indonesia lacks such mechanisms.
Applying liquid fertilizers at sea, could supply nutrient at a local scale. Liquid fertilizer has successfully enhanced the growth of Gracillaria cultivated in controlled pond settings (Nasmia et al., 2021). Applying fertilizers to seaweed in the sea presents challenges due to inefficiencies related to the large seawater volume and water movements. Daily application is impractical in terms of time, technical considerations, and cost (Tahiluddin et al., 2022). Another method, soaking seaweed in water filled with fertilizers before cultivation, has been shown to lack effectiveness due to the need for sustained nutrient supply (Tuiyo & Pasisingi, 2023). The application of fertilizer in seaweed cultivation has, to date, been predominantly directed towards small-scale or controlled environments such as tanks and ponds (Nasmia et al., 2021), while its application in open environments such as coastal seas remain extremely limited. Therefore, this study conducted an experimental trial of a slow-release solid organic fertilizer application method to support seaweed mariculture when low nutrient conditions occur. The purpose of this study was to determine the effectiveness of a novel method for applying fertilizer as a solution to low production of K. alvarezii seaweed farming due to seawater nutrient deficiency.
Materials and Methods
K. alvarezii was sourced from the same seed bank for all treatments, ensuring consistency in age and strain. Experiment seaweed were visually inspected for health and disease-free status before cultivation. The slow-release solid organic fertilizer was prepared using vermicompost, agar powder from Gracilaria sp., and freshwater, with vermicompost containing N-NH4 at 0.18%, N-NO3 at 0.19%, and PO4 at 0.34%. Aliquots of this mixture were placed within high-density polyethylene (HDPE) plastic tubes. These tubes measured 7 cm in diameter and 60 cm in length with an approximate volume of 2.31 L. Holes (3 cm diameter) were drilled in the tubes to facilitate the gradual release of nutrients. The treatments were different number of holes in the tubes (2, 4, and 6), thus varying the fertilizer release rate.
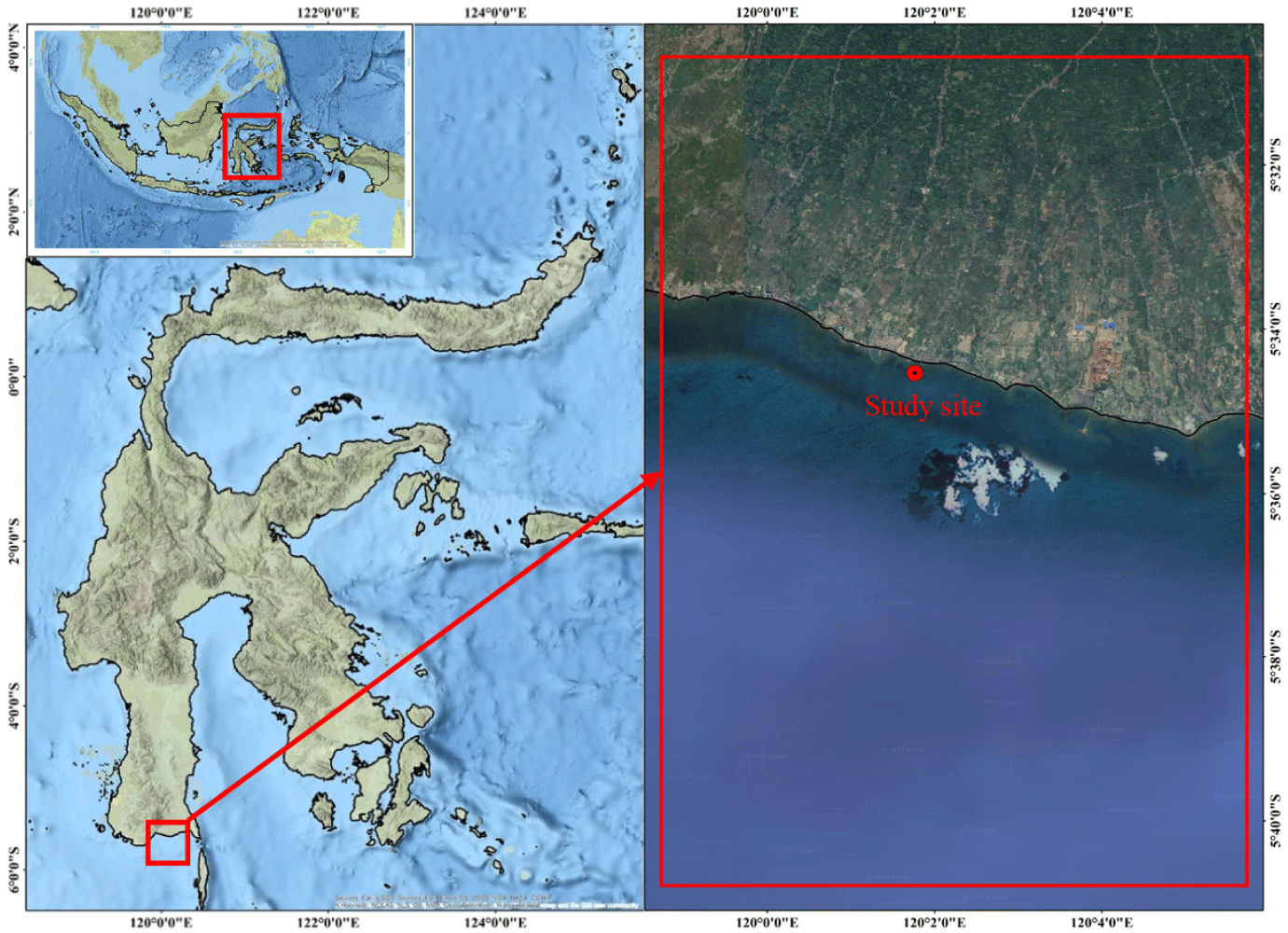
This research was conducted from 3 October to 7 November 2021 during a period of observed reduced nutrient availability in the seaweed farming area, indicated by low crop yields and instances of crop failure. The research location was in the coastal area of Bantaeng Regency (120° 1’ 45.8” E, 5° 34’ 32.2” S), one of the top seaweed-producing regions in South Sulawesi, Indonesia (Fig. 1). The coastal waters of the study area is characterized as open waters directly influenced by the Indonesian throughflow current conveying water from the Pacific Ocean via the Makassar Strait. This geographical interconnection significantly affects the nutrient inputs required for seaweed farming in this area.
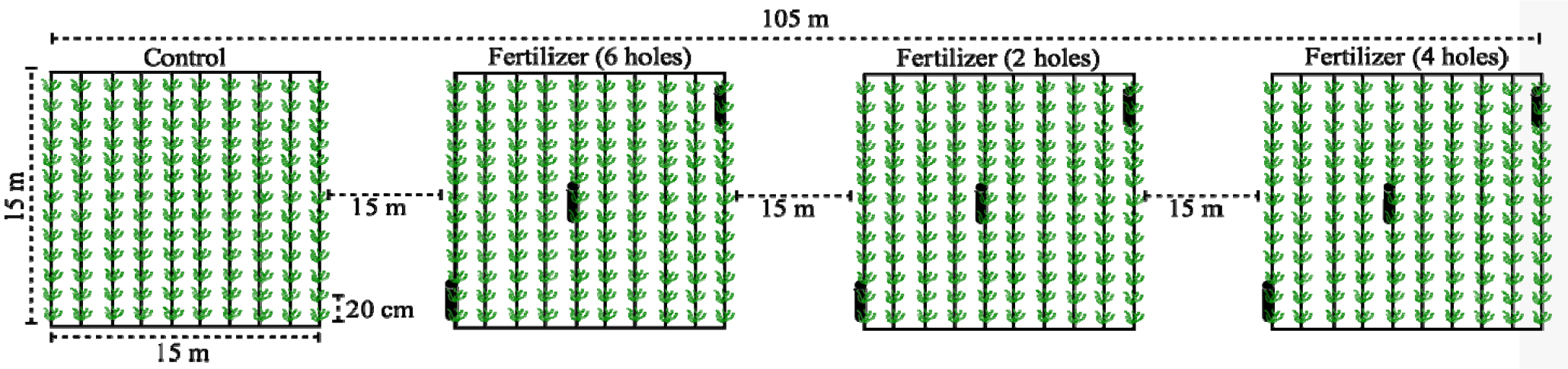
The Kappaphycus cultivation method used in this study followed the floating long-line method commonly employed by local farmers. The experimental setup included four seaweed cultivation plots, each measuring 15 × 15 m and collectively spanning an area of 225 m², with main lines constructed from jute rope. Each plot contained ten lines made of nylon ropes, locally known as “tali ris bentangan”, each 15 m long, with a spacing of 1.5 m between lines. Kappapychus seedlings were affixed to these ropes using smaller nylon ties, positioned 20 cm apart, resulting in 75 ties per line. Solid organic fertilizers in tube were secured by tying them to the Kappaphycus cultivation line, specifically at the first, fifth, and tenth lines in each plot. Three plots were fertilizer treatments and the fourth plot was designated as a control (no fertilizer treatment). This arrangement facilitated a direct comparison between fertilized and unfertilized seaweed growth. The cultivation plots were strategically positioned 15 m apart to minimize potential cross-interference while ensuring similar environmental conditions for all experimental units. This separation was crucial to isolate the effects of each fertilizer treatment, allowing for an accurate assessment of treatment impact compared to the natural seaweed growth observed in the control area (Fig. 2).
Data were collected weekly for six weeks during the cultivation period from 3 October to 7 November 2021. Environmental parameters measured in the field at the cultivation plots included water depth using a calibrated rope line, current velocity using a current meter, visibility using a Secchi disk; water temperature, turbidity, DO, pH and salinity using a water quality checker. Water samples were collected to measure ammonium (NH4+), NO3– and NO3– concentrations at the Chemical Oceanography Laboratory, Faculty of Marine Science and Fisheries, Hasanuddin University. Seaweed samples were randomly taken from one of the ties within seaweed lines from each plot. These samples were weighed to measure the absolute growth in seaweed biomass (weight gain), defined as the weight at the end minus the weight at the beginning of the cultivation period. Absolute growth was used as it provides a direct measurement of biomass increase during the cultivation period, which is critical for assessing total production in seaweed farming systems (Nasmia, 2021). The absolute growth of Kappaphycus seaweed was calculated using the formula:
where:
W = absolute growth in seaweed biomass (g)
Wt = weight of seaweed at time t (g)
Wo = initial weight of seaweed (g)
A one-way analysis of variance (ANOVA) was conducted to evaluate the effects of slow-release solid organic fertilizer on the average growth biomass and absolute growth of K. alvarezii over a six-week cultivation period. Prior to the analysis, tests for normality and homogeneity of variance were conducted to ensure the data met the assumptions for ANOVA. In cases where significant differences were observed (p < 0.05), post hoc tests were performed to identify pairwise differences between groups. The analysis compared the mean values for average growth biomass and absolute growth rate among four groups: a control group and three treatment groups with different fertilizer release rates. Each treatment group, including the control, was measured in three replicates.
Results
The weekly measurements of environmental parameters during the study are shown in Table 1. Most parameters remained within recommended ranges throughout the study period. The maximum water depth at the study site was 500 cm, and seaweed was attached to longlines with a planting depth of around 30–60 cm below the water surface. The highest turbidity occurred in the fifth week (11.7–13.78 NTU), while the lowest was in the first week (3.76–6.73 NTU). Salinity ranged from 17–33 ppt with the lowest salinity observed during heavy rain. The pH values were consistent across treatments, and remained within the range of 7.27–7.62 during the study.
| Parameter | Unit | Recorded range | Recommended Range | References |
|---|---|---|---|---|
| Planting depth | cm | 30–60 | 50–80 | Munisamy et al. (2023); Xu et al. (2018) |
| Visibility | cm | 293–310 | > 400 | Ya’la (2023) |
| Temperature | °C | 28–31 | 28–31 | Syahrul et al. (2023) |
| Current velocity | m/s | 0.03–0.06 | 0.25–0.35 | Savvashe et al. (2021); Zhu et al. (2021) |
| Turbidity | NTU | 3.76–13.78 | < 9 | Hasriah et al. (2019) |
| Salinity | ppt | 17–33 | 33–35 | Oedjoe et al. (2022) |
| pH | 7.27–7.62 | 6–9 | Labenua & Aris (2021) |
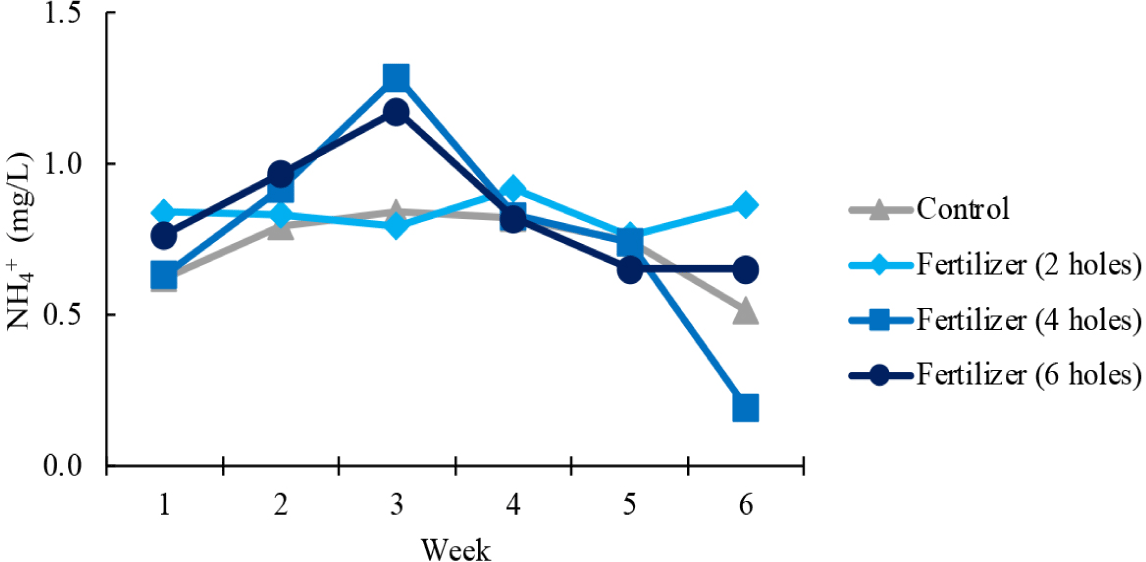
The application of organic fertilizers aimed to enhance nutrient levels in the waters, in particular the NH4+, NO3– and phosphate concentrations. NH4+ concentration varied between treatments, i.e., with the number of holes in the plastic fertilizer tubes (Fig. 3). The concentrations remained more stable under the treatment with two holes in the tube compared to the other treatments. In the four and six hole treatments, there was an initial increase in NH4+ concentration in the first three weeks, followed by a subsequent decline with the lowest concentrations from week 3 to week 6. The concentration remained low in the control plot throughout the 6-week study period.
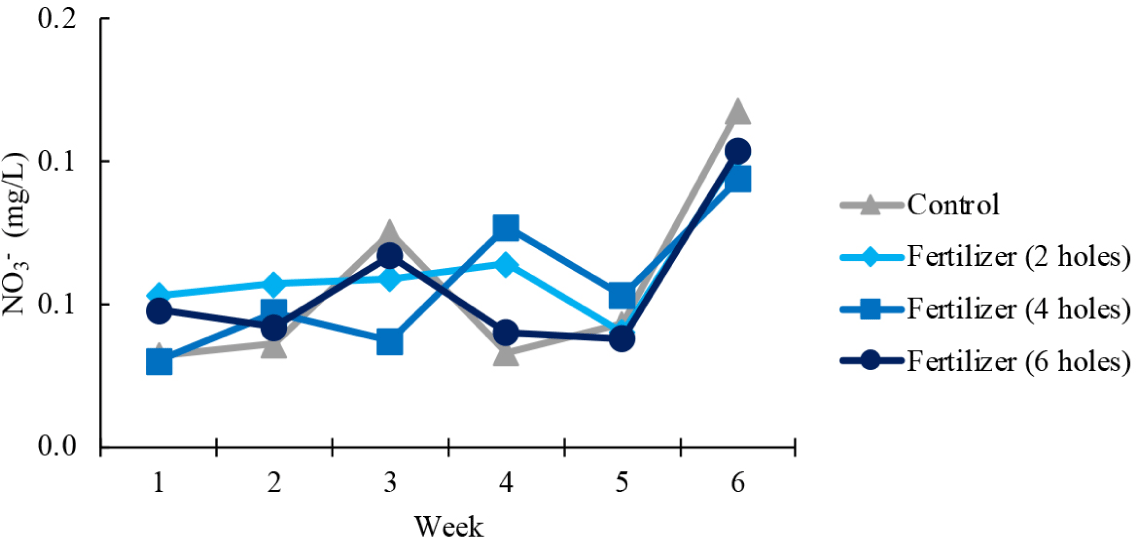
NO3– concentrations fluctuated in all treatments (Fig. 4). Despite these fluctuations, all treatments remained within a similar range for the first five weeks. In week 6, all treatments displayed an increase in NO3- concentration.
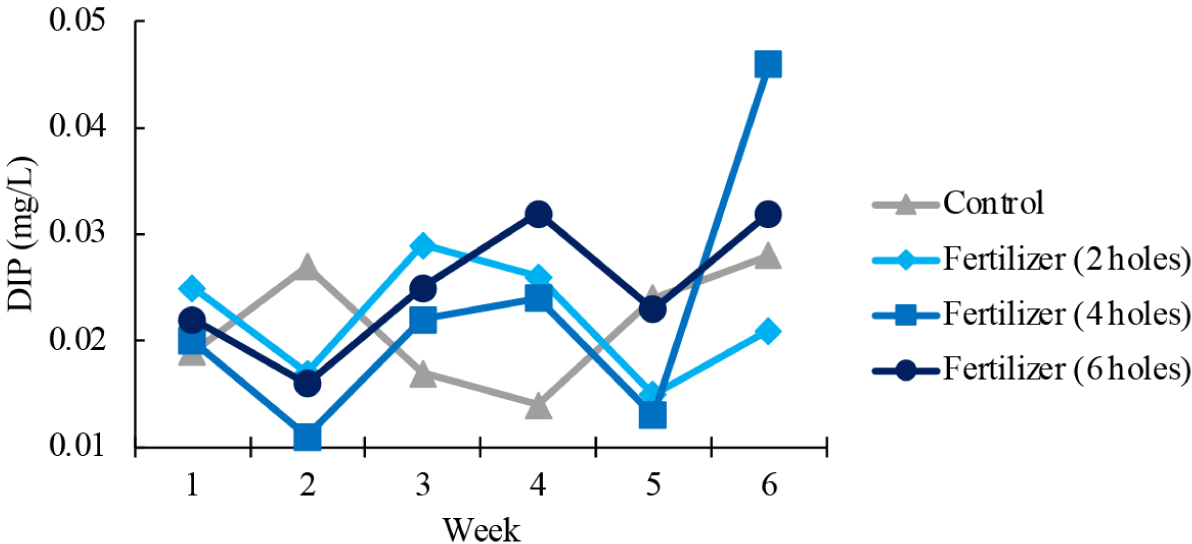
Dissolved inorganic phosphate (DIP) concentrations fluctuated during the study period in all treatment plots (Fig. 5). While the control saw a rise to 0.027 mg/L in the second week, DIP concentration decreased under all fertilized treatments. While fluctuations continued, DIP concentration was highest (0.046 mg/L) under the 4-hole treatment at the end of the cultivation period in the 6th week.
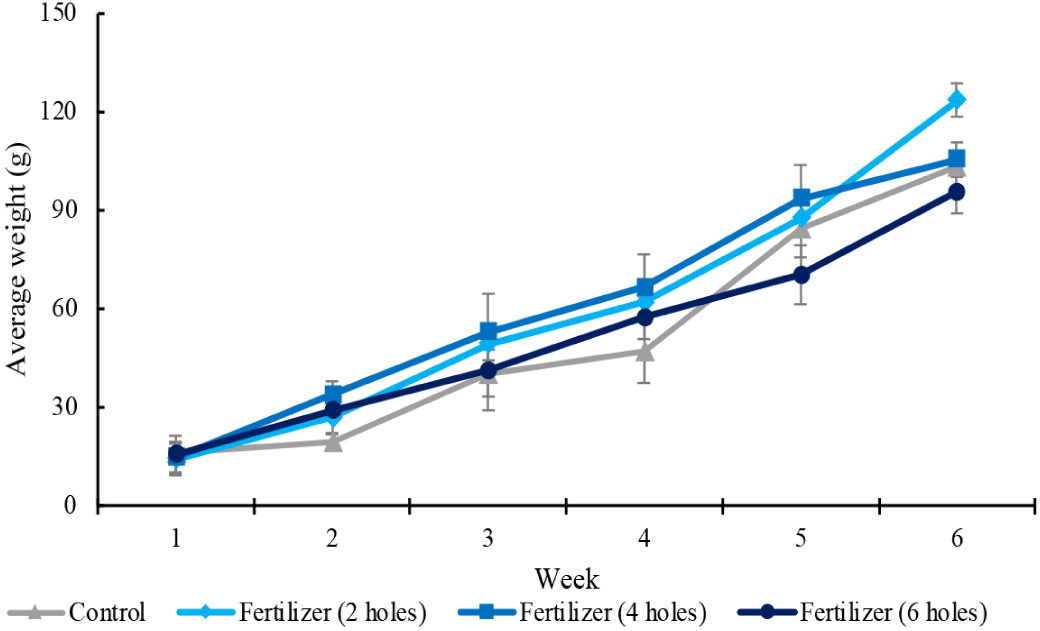
The ANOVA test results indicated no statistically significant differences (p > 0.05) in biomass growth among the treatment groups. All treatments showed an increase in biomass over time, with variations in growth patterns observed (Fig. 6). The four-hole treatment had the highest mean weight gain during the first five weeks, while by week 6, the two-hole treatment resulted in the highest final biomass. The six-hole treatment exhibited lower growth compared to the two-hole and four-hole treatments. The control group, which did not receive any fertilizer, consistently had the lowest biomass throughout the study. Although the two-hole and four-hole treatments resulted in higher mean biomass than the control, the variability within the data contributed to the lack of statistical significance.
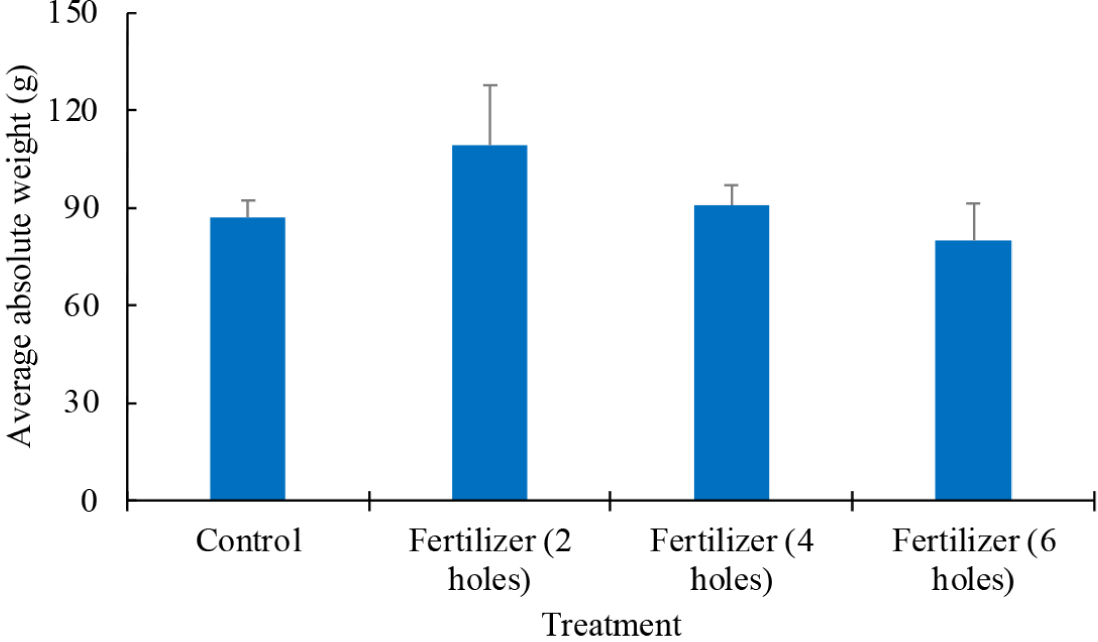
For the absolute growth rates, the ANOVA test results also indicated no statistically significant differences among the treatments (p > 0.05). The control group showed the lowest average growth rate at at 87 ± 3 g, while the two-hole treatment exhibited the highest mean growth rate at 109 ± 11 g. The four-hole treatment achieved an average growth rate of 91 ± 3 g, and the six-hole treatment resulted in an average growth rate of 80 ± 7 g. Despite these differences in average values, the statistical analysis confirmed no significant variations among the treatments.
Discussion
The environmental parameters measured in this study were largely within the ranges considered optimal or recommended for eucheumatoid seaweed growth. Planting depth, water clarity, temperature, current speed, salinity, and pH remained conducive to seaweed cultivation across all treatments. The planting depths observed in this study fell within the range identified by Munisamy et al. (2023) as ideal, and adequate sunlight penetration was ensured by good water clarity, which is crucial for photosynthesis (Revilla-Lovano et al., 2021). This reflects the suitability of the experimental conditions for supporting seaweed growth.
Temperature fluctuations during the study remained within the favorable range of 28–31°C, as reported by Syahrul et al. (2023). This is critical for maintaining enzymatic activity and photosynthetic efficiency in seaweed (Han et al., 2022). Similarly, current speeds were within the ideal range of 0.25–0.35 m/s for nutrient transport and oxygenation, facilitating nutrient absorption and preventing sedimentation on the thalli (Hayashi et al., 2020; Pang et al., 2015).
Salinity and pH levels also remained favorable for eucheumatoid seaweed growth. Salinity was within the optimal range of 33–35 ppt (Oedjoe et al., 2022), while pH values were maintained within levels conducive to photosynthesis and nutrient availability, as suggested by Vinuganesh et al. (2022). These findings confirm that the environmental parameters provided a stable and supportive backdrop for the treatments tested in this study.
The applications of fertilizers showed to support the nutrient level to be available for cultivated seaweed for intake for 6 weeks cultivation period. The supply of inorganic nutrients such as nitrogen and phosphate regulate the primary production in aquatic ecosystem (Alam et al., 2020). Seaweeds can utilize these nutrients in the process of photosynthesis (Narvarte et al., 2022). Nitrogen is absorbed by the seaweed in the form of NH4+ and NO3– which will be converted into organic compounds such as amino acids and other proteins to enable the growth of the seaweed thallus.
NH4+ concentrations were higher in the areas where fertilizer than in the control area, and the NH4+ concentrations remained stable over the 6-week study period for the 2-hole and 4-hole treatments. The application of fertilizers can ensure the availability of abundant nutrients to support photosynthesis, and thereby promote faster seaweed growth (Nasmia et al., 2021).
NO3– is also a nitrogenous compound needed for seaweed growth (Nasmia et al., 2021). NO3– concentrations fluctuated during the study, with no difference between the fertilized areas and the control area, remaining within the range considered suitable for seaweed growth. Fluctuations in DIP concentrations were observed across all treatments throughout the study. Specifically, there was an increase in DIP levels during week 6 for all treatments. This general increase can be assumed to be a consequence of environmental dynamics, one likely influencing factor being the high rainfall intensity during that period. The study location’s proximity to river estuaries along the coast likely contributed to the phosphate concentration, due to higher volumes of freshwater influx carrying organic materials. These findings are consistent with other studies, both locally (Lestari et al., 2021), indicating that phosphate concentrations tend to be higher in coastal areas close to river estuaries, a phenomenon attributed to the riverine transport of organic matter from inland areas to marine waters.
The availability of balanced nutrients between macro and micronutrients can promote good seaweed growth (de Oliveira Fernandes et al., 2017). The results from our study indicate that applying solid fertilizers with different release rates (controlled by the number of holes in the fertilizer containers) can affect both the nutrient levels in the seawater and seaweed growth. The treatments deploying fertilizer through tubes with two and four holes enhanced nutrient availability, thereby influencing seaweed growth patterns over the six-week study period. The steady increase in seaweed weight across all treatments, with a marked improvement in the groups with two and four holes, indicates the potential benefits of such fertilizer application.
With respect to the relative effectiveness of the fertilizer treatments compared to the control in terms of net weight gain, we observed that, even though the differences were not statistically significant, the treatments with two and four holes gave higher net growth than the control (Fig. 7). This suggests that, while there was an observable trend towards improved growth with fertilizer application, the difference was not large and/or consistent enough to conclude with certainty that the fertilizer treatments with two and four holes positively influenced seaweed growth. The rapid nutrient release in the treatment with six holes, under which the container was empty by week 4, underscores the importance of optimizing fertilizer release rates for sustained growth. These findings, together with the statistical analysis, highlight the complex interplay between nutrient availability and seaweed growth, emphasizing the need for precise fertilizer release controlled by holes numbers and size to enhance cultivation outcomes.
The higher growth in the treatment with four holes until week 5, followed by a decline in the final week, points towards the importance of sustained nutrient release rates to support steady growth. The decline in growth rate corresponded with the depletion of the fertilizer, suggesting that, while the initial release rate supported substantial growth, its complete utilization (and therefore the lack of any further nutrient release) by week 5 limited further growth. Conversely, the two-hole treatment maintained a more prolonged nutrient release, not exhausting the fertilizer supply until the harvest in week 6. This sustained release resulted in the highest average weight gain in the final week, indicating a more consistent nutrient availability that favored optimal growth and higher biomass yield. The nutrients provided by the fertilizers towards the end of the cultivation period are important to the growth rate and the absolute seaweed weight at harvest time. The rapid nutrient release and subsequent depletion observed after just four weeks in the six-hole treatment highlight the critical need for precision in fertilizer release rates, emphasizing the importance of matching fertilizer release with the growth needs of seaweed.
The comparison with the control group, which received no fertilizer, further highlights the nuanced impact of fertilizer application on seaweed growth, and the influence of natural environmental fluctuations. Although the two and four-hole treatments achieved higher absolute growth, the lack of statistical significance points to the complex dynamics between added nutrients and growth outcomes. While applying solid organic fertilizer can influence growth trends, the variability inherent in biological systems and environmental conditions may dilute the measurable impact of such interventions. This is in accordance with the findings of a study by Banerjee et al. (2020) that the interplay of seawater physical and chemical properties with the dynamic nutrient needs of seaweeds can influence seaweed growth.
Conclusion
Our research on using slow-release solid organic fertilizer in seaweed cultivation indicates the potential of this approach to enhance growth, particularly in nutrient-deficient waters, as well as highlighting challenges to its effective application. The methods used to ensure fertilizer release, in this case the number of holes in the fertilizer tubes, will impact nutrient release rates and, consequently, the effects on seaweed growth. The treatments with more holes ran out of fertilizer before the end of the cultivation cycle, leading to a decline in the final week and lower harvested weight. In contrast, the two–hole treatment demonstrated sustained nutrient release over six weeks, underscoring the importance of optimizing fertilizer application volume and rate. Despite the promising indications, the lack of statistical significance between the absolute growth of treated and control groups underlines the complex relationships between nutrient supplementation and the environmental variables affecting seaweed growth. Nonetheless, the results suggest that such fertilizer application warrants further investigation. Future studies should examine how to sustain nutrient release and maintain to maintain elevated growth for the full duration of the 6-week seaweed cultivation cycle, and elucidate the interplay between environmental factors and fertilizer application techniques. For example, trials using a larger container with the four-hole setup that produced the highest seaweed growth until the fertilizer ran out. We recommend the application of slow-release organic fertilizer only under low nutrient conditions to prevent eutrophication. Additionally, it is essential that a relevant agency should conduct nutrient monitoring to ensure that the application of the fertilizer is appropriate and environmentally sustainable.
