Introduction
Growth and development in childhood are fundamental determinants of long-term health outcomes, well-being, and overall quality of life. Among various developmental indicators, linear height is often recognized as a critical marker reflecting a child’s growth trajectory (Lampl, 2020). While inherited traits such as parental stature, birth weight, and sex pre-dominantly guide growth potential, modifiable factors, including nutrition and general health status, also exert a substantial influence on actual growth outcomes (di Liegro et al., 2019; Gokhale & Kirschner, 2003; Park & Lee, 2023; Roberts et al., 2022).
In Korea, advances in nutrition and socioeconomic status over recent decades have contributed to notable increases in average child height. Nevertheless, concerns about short stature persist among parents, prompting many families to seek interventions aimed at supporting healthy growth (Cole & Mori, 2018; Yuen et al., 2023). Growth hormone (GH) therapy remains a standard option for children with growth-related concerns; however, its widespread use is limited by practical barriers such as cost, discomfort from daily injections, and potential side effects. Several adverse effects have been reported, including rash and pain at the injection site, prepubertal gynecomastia, arthralgia, edema, benign intracranial hypertension, and slipped capital femoral epiphysis (Souza & Collett-Solberg, 2011). These limitations have led to increased interest in identifying safe, natural alternatives that may encourage height gain in otherwise healthy children without pathological growth deficiencies.
Various natural products have been investigated for their potential to promote height growth, including the Astragalus extract mixture HT042, which has demonstrated clinical efficacy in children with mild short stature (Lee et al., 2018), as well as other herbal formulations such as Epimedium and Panax ginseng, which have shown positive effects on longitudinal bone growth in animal models (Lim & Lee, 2023). Among such alternatives, marine-derived ingredients have garnered attention for their potential to support bone growth. Fermented oyster extract (FGO), produced from Crassostrea gigas, contains nutrients critical for skeletal development and cellular metabolism, including omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]), minerals such as zinc and iron, glycogen, taurine, and glutamic acid (Dagorn et al., 2016; Ishida et al., 2022; Venugopal & Gopakumar, 2017). Glutamic acid, a key component of FGO, is a precursor of gamma-aminobutyric acid (GABA), which has been suggested to promote the secretion of growth hormone and insulin-like growth factor 1 (IGF-1), offering a potential hormonal pathway for enhancing linear growth (Powers, 2013). In addition, the fermentation process may increase the absorption and biological activity of these nutrients, further supporting their potential to promote healthy bone development (Athapaththu et al., 2021).
Animal studies have indicated that FGO possesses osteogenic and growth-promoting activities, including lengthening of the tibia, acceleration of longitudinal bone growth, and increases in circulating IGF-1 levels (Lee et al., 2020b; Molagoda et al., 2020). Additionally, FGO has been reported to up-regulate markers of osteogenesis such as runt-related transcription factor 2 (Runx2) and osteocalcin, along with enhancing alkaline phosphatase activity, reinforcing its potential contribution to bone formation and mineralization (Lee et al., 2020b; Molagoda et al., 2020).
This secondary analysis builds on data from a previously reported randomized controlled trial (RCT) evaluating the efficacy of FGO for promoting height in children with idiopathic or constitutional short stature (Jeong et al., 2021). While the initial publication demonstrated that FGO supplementation significantly increased height in children below the 25th percentile, defined by the 2017 Korean Children and Adolescents Growth Standards, it primarily relied on an intention-to-treat (ITT) analysis and did not explore whether baseline height percentiles influenced individual treatment responses (Jeong et al., 2021; KCDC & KPCA, 2017). Such an analysis is clinically important, as children at the lowest percentiles (e.g., below the 3rd) may have undiagnosed endocrine, genetic, or chronic conditions that could reduce or mask the effects of nutritional interventions, potentially leading to underestimation of FGO’s true efficacy in idiopathic short stature. Therefore, this secondary study applies a per-protocol (PP) analysis to more accurately assess FGO’s effectiveness among children who completed the intervention as planned and to determine whether treatment outcomes differ across specific baseline percentile subgroups.
To address this issue, the current analysis presents outcomes both for the entire cohort (children below the 25th percentile) and separately for those above the 3rd percentile (3rd–25th percentiles). This stratification allows a more nuanced assessment of how potential underlying pathological conditions could impact treatment efficacy. Further sub-group evaluations were performed within the idiopathic short stature range, dividing children into those between the 3rd to 10th percentiles and the 10th to 25th percentiles, under both ITT and PP analyses. These detailed subgroup assessments aim to better elucidate whether initial height status affects responsiveness to FGO. This stratification was selected based on clinical relevance. Children below the 3rd percentile are typically evaluated for possible pathological causes of short stature, such as endocrine or genetic conditions. Those between the 3rd and 25th percentiles are generally considered to have idiopathic or constitutional short stature, for whom GH treatment is not always indicated. Further dividing this range into 3rd to 10th and 10th to 25th percentiles allows for more detailed analysis, as these cutoffs are often used in clinical practice to monitor growth and guide treatment decisions (Cohen et al., 2008; Lee et al., 2018).
Therefore, this secondary analysis seeks to build on these stratified evaluations by clarifying the relationship between FGO supplementation and height gain across baseline growth percentiles in children with idiopathic short stature. By examining treatment responses within these defined subgroups, this study aims to provide more precise insights into which children may benefit most from FGO, thereby informing the development of safe, natural strategies to support healthy growth.
Materials and Methods
This secondary analysis was performed on data from the randomized, double-blind, placebo-controlled trial conducted at Korean Medicine Hospital of Pusan National University (IRB No. PNUH-IRB-2019-04-001), previously published by Jeong et al. (2021). The study followed the Declaration of Helsinki and Korean Good Clinical Practice guidelines, and informed consent was obtained from all participants and their guardians.
The eligibility, randomization, intervention dosing (500 mg/day of FGO or placebo for 24 weeks), and clinical visit schedule matched those detailed in the original trial (Jeong et al., 2021). Baseline measurements, growth evaluations, dietary surveys, and safety monitoring followed the same protocol to ensure consistency. Details on adherence checks and laboratory analyses can be found in the original publication.
For statistical analysis, this secondary evaluation applied updated methods to examine treatment effects in subgroups defined by baseline height percentiles (below 3rd, 3rd–10th, and 10th–25th). Analyses for the overall below the 25th percentile group used the same approach described in the primary study, while subgroup-specific analyses were performed using newly applied statistical procedures.
Descriptive statistics summarized baseline characteristics. Between-group comparisons were conducted using independent t-tests or Mann–Whitney U tests, selected based on data distribution. Within-group changes from baseline were assessed with paired t-tests or Wilcoxon signed-rank tests. A two-sided p-value < 0.05 was considered statistically significant. Missing values in the ITT population were imputed using the last observation carried forward (LOCF) method.
General statistical procedures were performed using SPSS Statistics version 26.0 (IBM, Armonk, NY, USA), while additional subgroup modeling and visualizations were completed with R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). Safety monitoring procedures were identical to those in the original protocol (Jeong et al., 2021).
Results
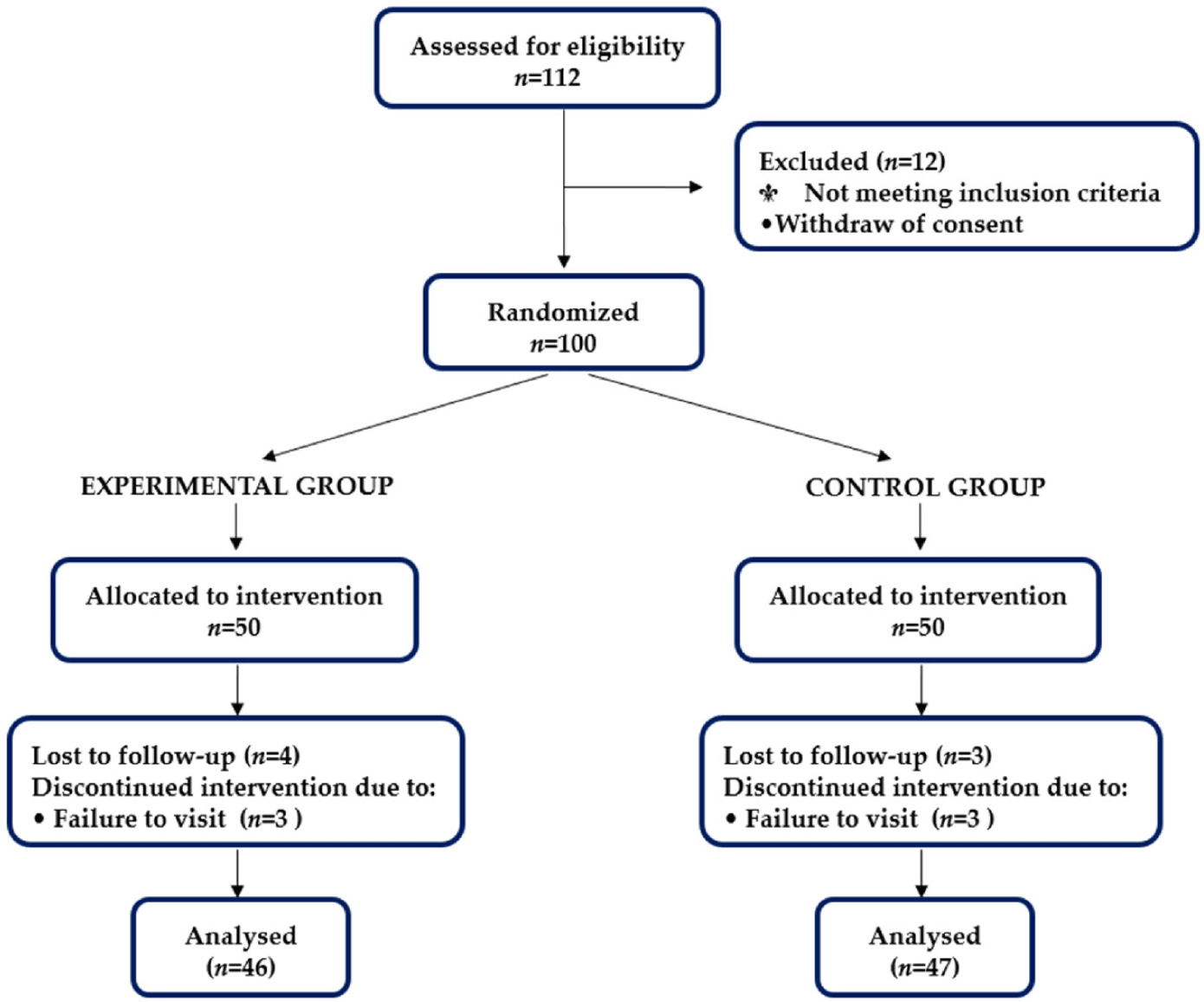
This secondary analysis is based on the baseline cohort from a previously published randomized controlled trial investigating FGO supplementation in children aged 6–11 years with idiopathic short stature (Jeong et al., 2021). Of the 112 children screened, 100 met the eligibility criteria and were randomly assigned to the experimental group (EG; n = 50) or the control group (CG; n = 50). During follow-up, seven participants (EG: n=4; CG: n = 3) discontinued participation due to consent withdrawal (n = 4) or missed visits (n = 3), resulting in 93 children (52 boys, 41 girls) who completed the study and were included in the PP analysis (Fig. 1). Specifically, 46 children in the EG and 47 in the CG adhered to the protocol and were analyzed accordingly.
Baseline characteristics, including age, height, and key clinical measures, were generally comparable between the EG and CG. A statistically significant difference in weight emerged only within the PP set, while the ITT population exhibited no such imbalance, indicating effective randomization. This weight difference in the PP analysis was attributed to post-randomization exclusions and was not expected to influence height outcomes, as baseline weight was not associated with treatment response. Additional baseline characteristics stratified by gender are presented in Table 1.
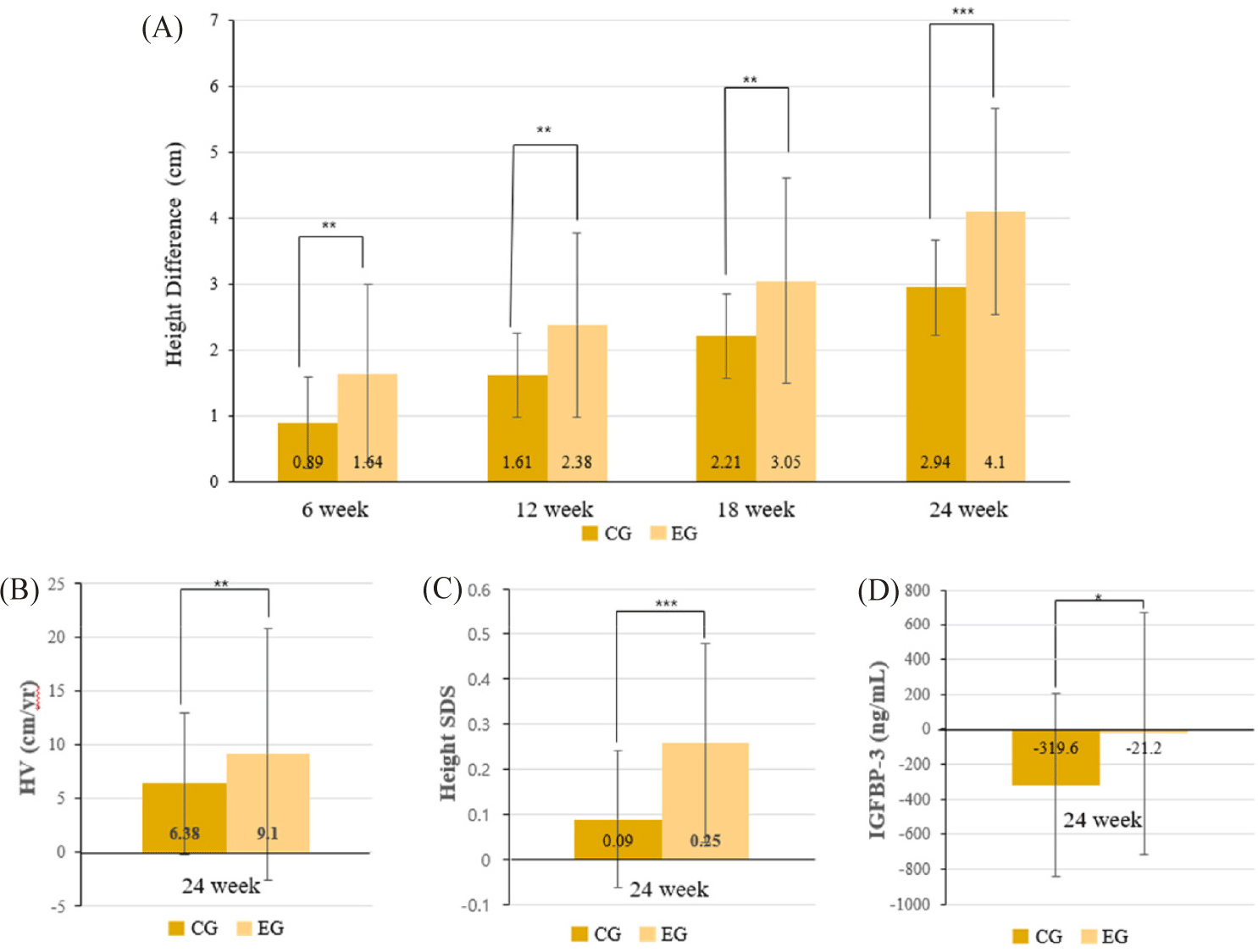
The primary efficacy outcome was the change in height after 24 weeks of intervention. In the PP population comprising children with baseline heights below the 25th percentile, the EG (n = 47) exhibited significantly greater height gain compared to the CG (n = 46) after 24 weeks of intervention. The mean height gain was 4.10 ± 1.57 cm in the EG, compared to 2.94 ± 0.73 cm in the CG (mean difference: 1.16 cm; p < 0.001). Within-group changes were also statistically significant for both groups (p < 0.001) (Table 2). Visual representations of these findings are provided in Fig. 2.
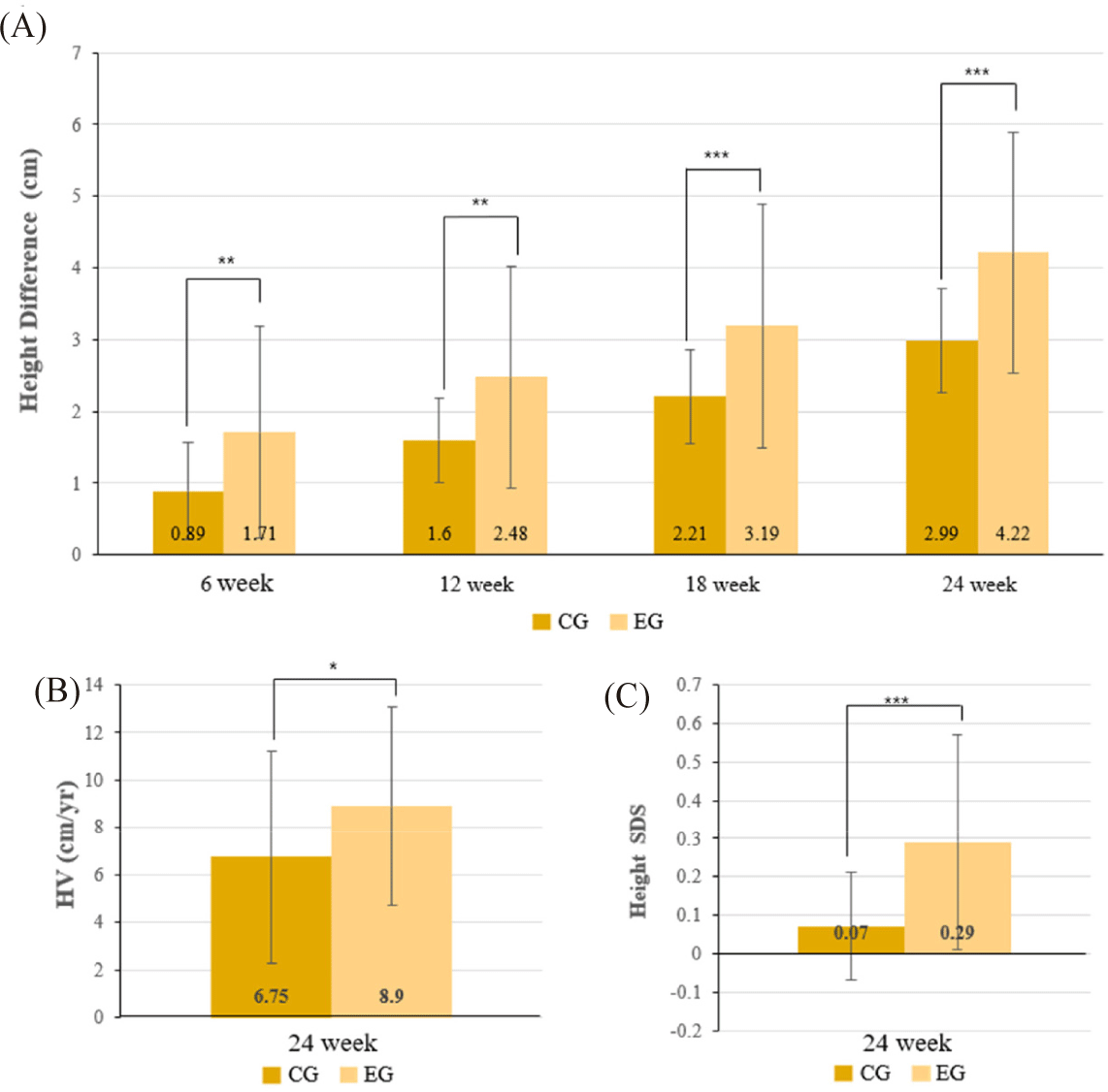
Given that children below the 3rd percentile are considered to have pathological short stature, additional analyses were conducted after excluding these participants. In the PP subgroup of children between the 3rd and 25th percentiles, the EG exhibited a significantly greater mean height gain of 4.22 ± 1.68 cm compared to 2.99 ± 0.72 cm in the CG, with a mean difference of 1.23 cm (p < 0.001) (Table 3). The mean height gain in the EG was greater in this subgroup than in the overall below the 25th percentile population, indicating a stronger treatment response after excluding children with extreme short stature. This trend is further illustrated in Fig. 3A.
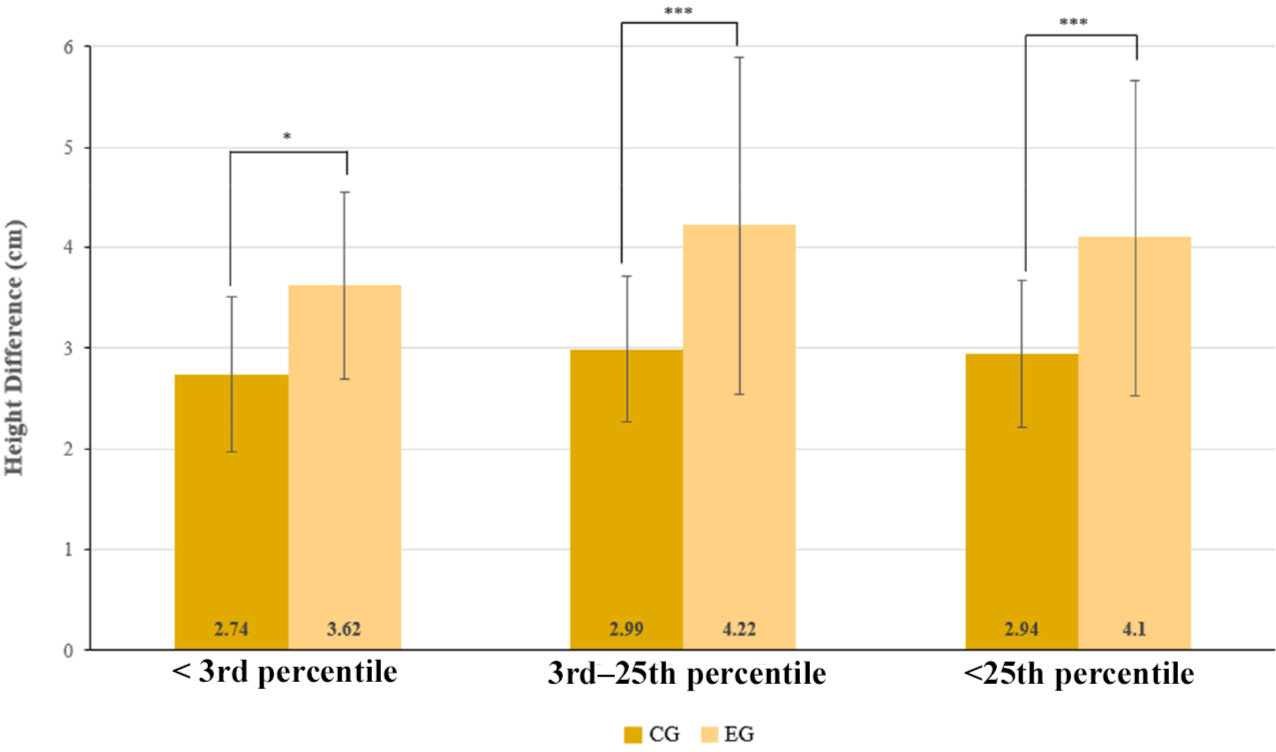
Further analysis stratified participants by baseline height percentiles. Among children between the 3rd and 10th percentiles, the mean height gain was 4.26±1.91 cm in the EG and 3.00 ± 0.82 cm in the CG, with a between-group difference of 1.26 cm (p = 0.012) (Table 4). In the 10th to 25th percentile subgroup, the EG showed a mean increase of 3.90 ± 1.91 cm, while the CG exhibited 3.08 ± 0.76 cm, resulting in a difference of 1.22 cm (p < 0.001) (Table 4). Among participants below the 3rd percentile, the mean height gain was 3.62 ± 0.93 cm in the EG and 2.74 ± 0.77 cm in the CG, with a between-group difference of 0.88 cm (p = 0.044) (Table 4). A visual comparison of height gain among the below 3rd percentile, 3rd to 25th percentile, and below 25th percentile subgroups is provided in Fig. 4.
Results from the ITT analysis were consistent with those of the PP analysis, displaying similar patterns of height gain and statistical significance. Detailed ITT results can be found in the Supplementary Tables S1–S6.
Height velocity (HV) significantly increased in the EG compared to the CG over the 24-week period. In below the 25th percentile population, HV was 9.10 ± 4.49 cm/year in the EG and 6.32 ± 4.47 cm/year in the CG (p = 0.004) as shown in Table 5 and visually illustrated in Fig. 2B. Among children between the 3rd and 25th percentiles, HV was 8.90 ± 4.19 cm/year in the EG and 6.75 ± 4.45 cm/year in the CG (p = 0.035) (Table 6, Fig. 3B). Subgroup analysis showed similar trends: between the 3rd and 10th percentiles, HV was 9.45 ± 4.51 cm/year in the EG and 7.24 ± 5.13 cm/year in the CG; between the 10th and 25th percentiles, HV was 8.67 ± 4.12 cm/year in the EG and 6.21 ± 3.63 cm/year in the CG. In participants below the 3rd percentile, HV was 9.92 ± 5.77 cm/year in the EG versus 4.53 ± 4.33 cm/year in the CG (p = 0.039) (Supplementary Tables S3, S6 and S7).
Height standard deviation score (SDS), improved significantly in the EG compared to the CG across all height percentile subgroups. In below the 25th percentile group, the mean change in height SDS (ΔSDS) was 0.27 ± 0.26 in the EG and 0.08 ± 0.14 in the CG (p < 0.001) corresponding to Table 5 and Fig. 2C. A similar trend was observed in the 3rd to 25th percentile subgroup, with ΔSDS values of 0.29 ± 0.28 (EG) and 0.07 ± 0.14 (CG) (p < 0.001) (Table 6, Fig. 3C). Within the 3rd to 10th percentile subgroup, ΔSDS was 0.27 ± 0.30 in the EG and 0.08 ± 0.14 in the CG (p = 0.008), while in the 10th to 25th percentile subgroup, the respective values were 0.25 ± 0.27 and 0.09 ± 0.17 (p = 0.026). In below the 3rd percentile subgroup, the EG demonstrated a ΔSDS of 0.20 ± 0.16 compared to 0.10 ± 0.15 in the CG (p = 0.099) (Supplementary Tables S3, S6 and S7).
Endocrine markers showed modest changes over the course of the study. GH and IGF-1 levels decreased in both groups without significant between-group differences (GH: −1.50 ± 3.57 ng/mL [EG] vs. −0.39 ± 3.53 ng/mL [CG], p = 0.135; IGF-1: −11.62 ± 46.58 ng/mL [EG] vs. −19.50 ± 31.76 ng/mL [CG], p = 0.342) (Table 5 and 6). Nevertheless, the magnitude of decline tended to be smaller in the EG across all subgroups, suggesting a relatively preserved endocrine response. In contrast, insulin-like growth factor-binding protein 3 (IGFBP-3) levels were significantly better maintained in the EG, showing a smaller reduction compared to the CG (−23.04 ± 723.45 ng/mL vs. −340.00 ± 534.16 ng/mL, p = 0.019) (Tables 5, 6, and Fig. 2D). This trend was consistently observed across all subgroups, although not statistically significant at the individual subgroup level (Supplementary Tables S3, S6 and S7).
Bone age (BA) progression over the 24-week period did not show significant differences between groups across any percentile subgroup. In below the 25th percentile group, the mean increase in BA was 8.33 ± 10.07 months in the EG and 8.57 ± 6.75 months in the CG (p = 0.709; Table 5). Similar findings were observed in the 3rd to 25th, 3rd to 10th, 10th to 25th, and below the 3rd percentile subgroups (Table 6, Supplementary Table S3 and S4). Although no statistically significant differences were observed, this result should be interpreted considering the baseline imbalance in BA between groups at the start of the study. Such initial differences may have limited the ability to detect treatment-related changes in BA during follow-up. Therefore, while FGO supplementation consistently improved linear growth, its effect on skeletal maturation remains inconclusive and should be further explored under more balanced baseline conditions.
Bone turnover markers, including serum osteocalcin (OC) and urinary deoxypyridinoline (DPD), showed minor within-group changes, with no significant differences be-tween groups. Consistent trends across all subgroups indicate that linear growth occurred without abnormal bone remodeling or resorption (Tables 5, 6, Supplementary Tables S3, S6 and S7).
Results from the ITT analysis were consistent with those of the PP analysis, displaying similar patterns of height gain and statistical significance. Detailed ITT results can be found in the Supplementary Table S1–S5.
Safety was evaluated in all 100 participants aged 6 to 11 years who received at least one dose of the study intervention (FGO or placebo). Because this secondary analysis used the same cohort and monitoring protocol as the primary clinical trial, safety outcomes were consistent with those previously reported (KCDC & KPCA, 2017). Throughout the 24-week study period, no serious adverse events (AEs) were observed in either group. A single mild AE, urticaria, occurred in one participant in the EG (1%), which resolved spontaneously without medical intervention and did not lead to study discontinuation or unblinding. The event was reviewed by pediatric investigators and deemed unrelated to the intervention.
Routine safety assessments, including hematologic and biochemical laboratory tests, revealed no clinically significant abnormalities. Vital signs and findings from physical examinations also remained within normal limits throughout the study period. These results indicate that daily administration of 500 mg of fermented oyster extract was safe and well tolerated in children aged 6 to 11 years.
Discussion
This randomized, placebo-controlled clinical trial demonstrated that FGO supplementation significantly increased height in children with short stature. In the PP population, the growth-promoting effect was consistently observed among children between the 3rd and 25th percentiles (CG: n = 38; EG: n = 37), with similar magnitudes of improvement in the 3rd to 10th (CG: n = 20; EG: n = 11) and 10th to 25th (CG: n = 18; EG: n = 26) percentile subgroups. Although subgroup analyses revealed modest absolute differences (approximately 1.2–1.3 cm) between the EG and CG, the exclusion of children below the 3rd percentile (CG: n = 9; EG: n = 9) clarified this consistency. Children in this subgroup often present with underlying medical conditions such as growth hormone deficiency, genetic syndromes, or chronic illnesses. In clinical practice, they are typically evaluated for specific diagnoses and may require pharmacological treatment rather than nutritional support alone. Including them in the overall analysis could have obscured the clearer treatment effects observed in children with idiopathic short stature. A comparable treatment effect was also observed among children below the 25th percentile (CG: n = 47; EG: n = 46). These findings suggest that FGO may be broadly effective in children with idiopathic mild short stature, with consistent efficacy across percentile strata.
Children in below the3rd percentile showed limited response to FGO, with a height increase of 3.62 cm over 24 weeks compared to 2.74 cm in the CG (Δ = 0.88 cm, p = 0.044), and this gain was not significantly different from other groups. This subgroup likely includes individuals with pathological causes of growth delay such as growth hormone deficiency, syndromic conditions, or chronic systemic diseases, which require pharmacologic therapy rather than nutritional support for meaningful improvement (Cohen et al., 2008). In contrast, children in the 3rd to 25th percentile, who are more likely to have idiopathic short stature demonstrated a clearer and more consistent response to FGO.
In this stratified analysis, children in the 3rd to 10th percentile grew an average of 4.26 cm in the EG compared to 3.00 cm in the CG (Δ = 1.26 cm, p = 0.012), while those in the 10th to 25th percentile grew 4.20 cm versus 2.98 cm (Δ = 1.22 cm, p < 0.001). The 3rd to 25th percentile group exhibited a net gain of 1.23 cm. These findings suggest a consistent growth-promoting effect of FGO in children with idiopathic short stature, regardless of more detailed stratification within this percentile band.
The underlying biological mechanism may involve modulation of the IGF-1 axis, particularly through stabilization by IGFBP-3. While serum IGF-1 levels decreased in both groups over the course of the study, the reduction was smaller in the EG, although this difference was not statistically significant. This trend is consistent with the age-dependent downregulation of IGF-1 during mid-childhood (Juul, 2003). In contrast, IGFBP-3 levels in the subgroup below the 25th percentile were significantly better maintained in the EG compared to the CG (p = 0.018). IGFBP-3 is known to prolong the half-life of circulating IGF-1 and facilitate its delivery to target tissues through the formation of a ternary complex with the acid-labile subunit (Firth & Baxter, 2002). Therefore, the observed IGFBP-3 pattern may reflect a more stable IGF-1 signaling environment in the EG, despite no significant change in total IGF-1 levels.
IGF-1 concentrations in children are known to fluctuate due to diurnal rhythms, nutritional status, and seasonal variation. In contrast, IGFBP-3 has a longer half-life and is more stable, making it a more reliable biomarker for assessing growth-related interventions. This may explain why IGFBP-3 showed a clearer response than IGF-1 (Blum & Ranke, 1990; Juul et al., 1995). In addition, IGFBP-3 has been shown to enhance IGF-1 receptor binding and intracellular signaling in chondrocytes, supporting its role in promoting longitudinal bone growth (Lee et al., 2020a). Notably, previous in vitro studies using fermented oyster extract have demonstrated its direct impact on the GH–IGF axis. Specifically, FGO treatment in hepatic and osteoblast-like cells resulted in increased IGFBP-3 expression and enhanced phosphorylation of IGF-1 receptor substrates, suggesting improved post-receptor signaling efficiency (Lee et al., 2020a). These findings indicate that FGO may enhance IGF-1’s biological effects via improved stability and signaling efficacy through IGFBP-3. This mechanistic insight aligns well with the clinical observation that height increased alongside IGF-1 activity.
Bone metabolism markers in this study provide additional insights. Osteocalcin, a marker of osteoblast activity, increased significantly in both the experimental group and the control group. However, the greater magnitude of increase observed in the EG may suggest an enhanced osteogenic response to FGO supplementation. Meanwhile DPD, a marker of bone resorption, did not differ significantly between groups. This indicates that linear growth occurred through physiological osteoblastic activity without excessive bone remodeling or resorption. In addition, although bone age increased in the experimental group, it progressed in proportion to height gain and without significant elevation in sex hormones, suggesting physiologic catch-up growth rather than premature skeletal maturation. These findings align with previous reports that link nutrient driven linear growth with enhanced osteogenesis rather than increased bone turnover (Zhu et al., 2005).
In summary, while this trial demonstrated that FGO supplementation is both effective and safe for promoting height growth in children with idiopathic or constitutional short stature, it also suggests a possible association with IGF-1 signaling support, particularly in children below the 25th percentile, although no significant group-level differences were observed. This analysis represents a percentile-based re-evaluation of the original clinical trial, excluding participants below the 3rd height percentile to more precisely assess efficacy in children with mild short stature. These findings support FGO as a nutritional intervention that promotes growth without disrupting hormonal balance. Notably, the same dose and duration were applied in a subsequent clinical trial, allowing for continuity across studies and further validation of its effects. Further investigation, including sex stratified analysis and evaluation of sex hormone changes as conducted in the follow-up trial, is warranted to refine clinical applications and understand long term effects.
In conclusion, this clinical trial provides compelling evidence that daily FGO supplementation can effectively support height growth in children with idiopathic or constitutional short stature, particularly those between the 3rd and 25th percentiles. The consistent and statistically significant improvements in height, alongside favorable trends in bone formation and hormonal balance, highlight FGO as a promising and safe nutritional intervention.
