Introduction
An individual biological success is determined by the number of offspring they bear over their lifetime (Wootton, 1998). Studying the reproductive biology of fish species is essential for understanding their economic potential, culture techniques, and real fishery management (Doha & Hye, 1970). Various methods are used to assess the reproductive condition in fishes, including microscopic gonadal staging, oocyte size-frequency distribution, sex’s steroid measurement and gonadal indices (Lowerre-Barbieri et al., 2011). The weight of the gonad in relation to body weight is indicated as a percentage by the gonad-somatic index (GSI; Bagenal & Tesch, 1978). Fish reproductive seasonality can be reasonably inferred from monthly fluctuations in the GSI. According to Admassu et al. (2015), fecundity is a biological phenomenon that occurs in fish populations and is defined as the quality of ripe eggs discharged before spawning. One of the key biological facts considered to be necessary to explain the mathematical link between variations, length, and weight is the length-weight relationship. The variation in length-weight is determined by the trophic condition of a certain aquatic habitat as well as biotic and abiotic environmental factors. It is crucial to understand the reproductive biology of the fish species that are exploited in order to manage the fish population effectively. The life history of fish plays a crucial role in determining the reproductive capability of the unit stock by measuring its length at first maturity (L50). Fish biology can be impacted by a variety of factors, including stress, sex, season, food availability, fishing effort (mesh sizes), and other characteristics of the water quality (Tesfaye et al., 2016).
The threats to global freshwater biodiversity can be grouped under five interacting categories: overexploitation; water pollution; flow modification; destruction or degradation of habitat; and invasion by exotic species (Revenga et al., 2005). Habitat fragmentation is a form of degradation as it separates the habitat into isolated parts. Habitat degradation is brought about by an array of interacting factors. It may involve direct effects on the aquatic environment (such as excavation of river sand) or indirect impacts that result from changes within the drainage basin. For example, forest clearance is usually associated with changes in surface runoff and increased river sediment loads that can lead to habitat alterations such as shoreline erosion, smothering of littoral habitats, clogging of river bottoms or floodplain aggradation (Rahel, 2002). Freshwater fish populations face numerous threats that can significantly impact their reproductive success such as habitat destruction, overfishing, climate change and others. Addressing these threats is essential for protecting freshwater fish populations and ensuring their long-term survival. By taking a comprehensive approach to address these threats, we can help safeguard freshwater fish populations and ensure their continued survival for future generations.
The reproductive biology of each fish species plays a major role in fish resource management and conservation. Because of this, extensive research was carried out in Ethiopia’s natural lakes to manage and conserve fisheries resources in Lake Langano (Kebede et al., 2018); Lake Ziway (Abera et al., 2014); and Lake Koka (Tesfahun, 2018). However, in Tigray National Regional State few studies conducted on reproductive aspects of different fish species in Tekeze reservoir (Gebru, 2020; Teame et al., 2018) and in some tropical small earthen dams of Tigray (Dejenie et al., 2015). Yet, no research had been conducted on reproductive aspects of any fish species in Mihtsab-Azmati reservoir. Therefore, the present study was conducted to fill this gap and to provide useful information on some reproductive aspects of commercially important fishes that can contribute for food security and nutritional quality of food to be eaten by the local people around the reservoir.
Materials and Methods
Tigray is found in northern part of Ethiopia with a rugged terrain ranging between 400 to almost 4,000 masl, covering a total area of 50,079 km2 and it lies between latitudes 12°15’ and 14°57’N and longitudes 36°27’ to 39°59’E (CSA, 2006). Tigray National Regional State is bordered by Eritrea to the north, the Sudan to the west, and Amhara and Afar regions of Ethiopia to the south and east respectively (Fig 1). Mihtsab-Azmati reservoir is found in Baekel Tabia, Mereb Leak wereda, central zone of Tigray. This reservoir was constructed in 2014 with an actual water capacity of 34,000 mm3, and 56 m height and 450 m length. This reservoir was constructed mainly for irrigation purpose (TWRMEB, 2010).
At each sampling occasion, depth-integrated water samples (surface, middle, and just above the bottom) were collected at two littoral and two pelagic sampling sites with a heart-valve sampler (Silkeborg 8600, Denmark; volume: 3 L) between 9:00 and 10:30 am. Electrodes were used to measure the water quality parameters at each depth, and the mean value was calculated. With a portable meter WTW Multi 340 I electrode (Geotech Environmental, Denver, CO, USA), the following parameters were measured in-situ: temperature, conductivity, dissolved oxygen, and pH. In addition to measuring the conductivity of the combined samples, each of the three depths’ temperature, pH, and dissolved oxygen were recorded. Turbidity and the concentration of chlorophyll a were also measured in the pooled sample with a fluorometer (Turner Aquafluor 8000-001; Turner Designs, San Jose, CA, USA) (mean of three measurements) from the pooled sample. Depth was estimated with a pre-labeled rope, and water transparency was determined using a Secchi disc (diameter: 30 cm).
Fish samples were collected bi-monthly from two littoral and two pelagic sites using standardized experimental multi-mesh gillnets (28 m long, 3 m wide and composed of 7 frames of 4 m each with a different mesh size class ranging between 6.25 and 46 mm knot-to-knot) deployed in the late afternoon (5:00 pm) and retrieved the following morning (9:00 am). During fish specimen collection, total weight (TW; using digital balance) and total length (TL; using meter) were measured to the nearest 0.1 g and 0.1 cm, respectively. Sexes of individual fish specimens were differentiated visually only by dissecting 10% of the collected fishes. Maturity stages of fish species were assessed based on size, appearance, shape, texture and colour of the gonads depending on the maturity indexes of fish (Armstrong et al., 2004).
Sex ratio: The sex ratio is the proportion of females to males. Therefore, the sex ratio of selected fishes collected during the sampling period from Mihtsab-Azmati reservoir was determined using this formula:
The sex ratio (female:male) was calculated for the total sample. The chi-square test was employed to test whether the sex ratio varied from 1:1 in the total sample.
The weight of the gonad represented as a proportion of the somatic body weight is known as the gonado-somatic index. The time and frequency of the species’ yearly spawning have been ascertained by examining graphs of the mean monthly GSI against months (Bagenal & Tesch, 1978). The percentage of GSI was calculated from the gonad weight (g) and body weight (g) using the following formula:
Length-weight relationship: The relationship between TL and TW of the selected fish species was estimated by the power function formula of least squares regression (Bagenal & Tesch, 1978) as follows:
where TW is total weight in g, TL is total length in cm, and a and b is intercept and slope of the equation, respectively.
Condition factor (Fulton’s factor): This is used to determine the condition of fishes in their respective habitats. The individual TLs and TWs record was used to determine the condition factor of fish species. Fulton condition factor (FCF) was used to calculate from the following formula (Bagenal & Tesch, 1978):
where FCF is Fulton condition factor, TW is total weight in gram, TL is total length in centimeter.
Ethical considerations: The criteria for ethical treatment of animals were adhered to in the care and use of the experimental animals in this work. Ethiopia doesn’t have an ethical approval procedure for fishing data.
Data were analyzed using SPSS statistical software version 22 (SPSS, Chicago, IL, USA), and presented by various descriptive statistics. The sex ratio was calculated for the total period and also for individual months. The sex ratios obtained were tested using the χ2 (Chi-squared test) with a significance level of 5% to verify whether there was significant difference with the hypothesis of 1:1 ratio. Non-linear regression was used to establish the weight-length relationship. To ascertain whether the b values deviate substantially from 3.0, we employed the student’s t-test. One way analysis of variance (ANOVA) was also employed to analyze the monthly variation of GSI. Regression analysis was used to analyze the relationship between the TL, and the TW of the fishes. All the statistical analyses were considered at significance level of 5% (p < 0.05).
Results
The mean monthly values of limnological variables of Mihtsab-Azmati reservoir recorded within the study period are presented in (Table 1). The highest mean pH value (8.59 ± 0.08) and transparency (cm) (243.33 ± 3.54) were recorded in September 2019. The highest and the least mean values of water temperature were recorded in March (26.46 ± 0.14) and January 2019 (21.77 ± 1.11), respectively. Highest mean water turbidity was recorded in July 2019 (4.59 ± 0.82) and highest mean conductivity was recorded in January 2019 (276.50 ± 1.15). All the water quality variables were spatially significant difference (p < 0.05) except. However, temporally all the water quality variables were significant difference (p < 0.05; Table 1).
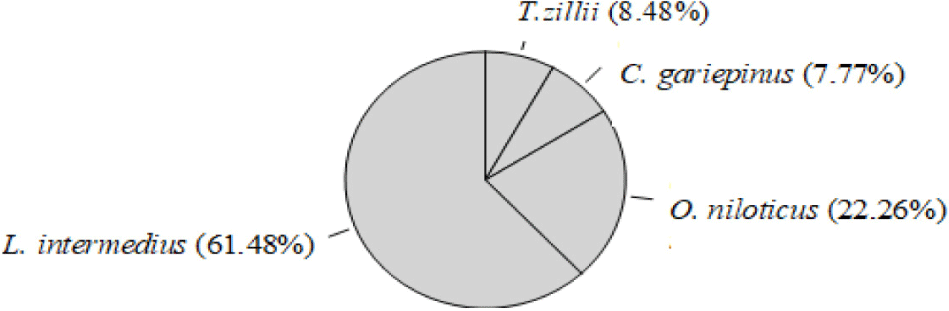
A total of 2,538 fishes were caught, out of this 283 fish specimens (72.08%; n = 204) males and (27.91%; n = 79) females were examined for analysis of reproductive biology of four species: Oreochromis niloticus, Clarias gariepinus, Labeobarbus intermedius and Tilapia zillii. These fish species belong to three families; Cyprinidae (L. intermedius), Cichlidae (O. niloticus and T. zillii) and Clariidae (C. gariepinus). Among the fish species L. intermedius was recorded as the dominant species (61.48%; Fig 2).
Males were more numerous than females for most fish species throughout the study period. The sex ratio for L. intermedius was significantly different (χ2 = 36.782, p < 0.05) from1:1. Sex ratio for O. niloticus did not show significant difference from1:1 except for November 2018. Generally, the total sex ratio was highly significant (χ2 = 7.000, p < 0.01) with predominance of males over females throughout the study period (Table 2).
Some fish reproductive aspects such as TL, TW, sex ratio, gonad weight, ovary weight and maturity stage were analyze spatialy. All the listed fish reproductive aspects were spatially significant difference (p < 0.05; Table 3).
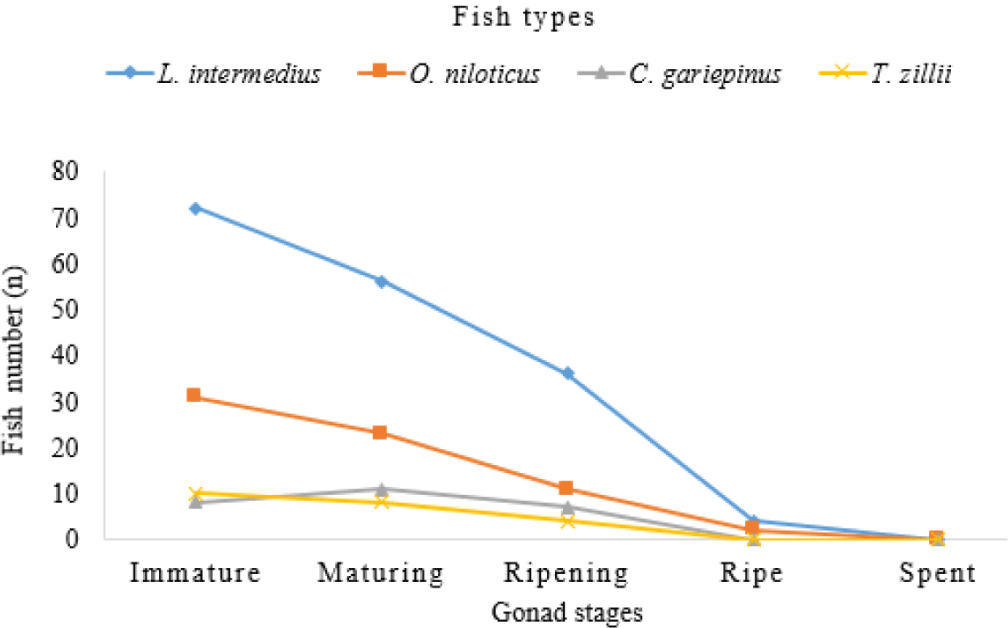
A total of 283 fish specimens were collected, out of which 121 (42.76%) were immature. Furthermore, 98 (34.63%) and 58 (20.49%) of the collected fish samples were under maturing and ripening stages of gonad, respectively. Only two percent (n = 6) of them were categorized under the gonad proportion of mature (gonad stages IV) but no fish species was recorded in stage V (Fig 3). Fish maturity stage was decreased starting from stage I (Immature) to stage V (spent) in all the identified selected fish species.
Among the different fish species collected and analyzed for reproductive aspect in Mihtsab-Azmati reservoir, L. intermedius recorded with highest percentage composition in immature stage (59.5%), maturing stage (57.14%), ripening stage (62.07%) and ripe stage (66.67%). However, C. gariepinus and T. zillii were recorded with lowest percentage composition in immature stage (6.61%) and maturing stage (8.16%), respectively (Table 4).
Depending on the size range the smallest sexually mature fish caught was 10 and 12 cm TL for males and females, respectively. The average length (L50) at which 50% of the female L. intermedius reached sexual maturity was 24.5 cm and 18.75 cm TL male and female, respectively. While 25 cm and 253 g of TL and TW, respectively were recorded sexually matured male O. niloticus and for smallest female sexually matured was recorded with 15 cm TL and 55 g TW. Accordingly, the smallest sexually matured male T. zillii in the study site was with 15 cm TL and 78 g TW and the average length (L50) at which 50% reached sexual maturity was 18.5 cm of TL. The smallest sexually matured male C. gariepinus was recorded with 32 cm TL and 224 g TW and the average length (L50) at which 50% reached sexual maturity was 44 cm and 837 g of TL and TW, respectively. But female C. gariepinus with 15 cm TL and 88 g TW were recorded with smallest sexually matured stages III to V.
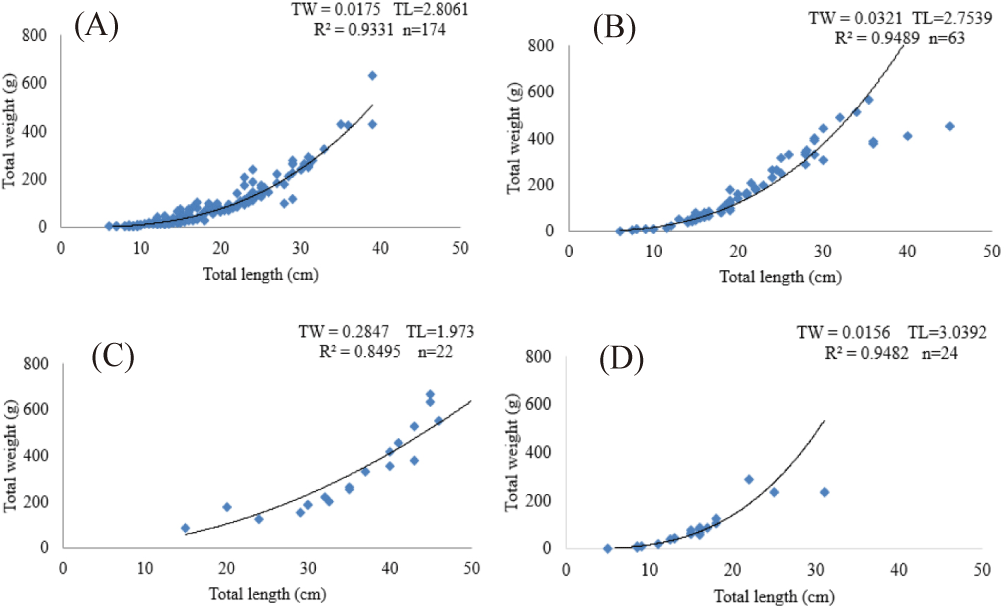
The relationship between TL and TW of the selected fish species were curvilinear. Therefore, the “b” values of both female and male exhibit negative allometric growth as the value of “b” was less than 3; but female T. zillii showed positive allometric growth (“b > 3”). The regression equation for female L. intermedius was expressed as TW = 0.0274TL2.6607 (R² = 0.918, n = 47) and for males it was TW = 0.015TL2.8552, (R² = 0.9392, n = 127). Furthermore, O. niloticus also showed curvilinear relationship between TL and TW and the line fitted to the data was described by the regression equation but was not statistically significant (p >0.05). The regression equation for males was expressed as TW = 0.0309TL2.7613 (R² = 0.9551, n = 42) and for females it was TW = 0.034TL2.7452, (R² = 0.9162, n = 21). The relationship between TL and TW for C. gariepinus were also curvilinear and statistically not significant (p >0.05; Fig 4).
Monthly mean GSI for O. niloticus is (mean 3.03 ± 0.98) and the highest GSI was observed in January 2019, while the least was observed in September 2019. Similarly, lowest (1.08) GSI value of C. gariepinus was recorded in July 2019. The highest percentage frequency of ripe C. gariepinus (50%) was recorded in March 2019. However, L. intermedius had GSI mean values (6.89 ± 1.44) with the highest and the lowest GSI value scored in January and July 2019, respectively. The highest percentage frequency of ripe L. intermedius (33.33%) was recorded in November 2018. The minimum and maximum GSI value of T. zillii was 0.11 and 13.33 (mean 3.85 ± 1.82). The highest GSI value of male T. zillii was recorded in January 2019 but the least was recorded in May 2019 (Fig 5).
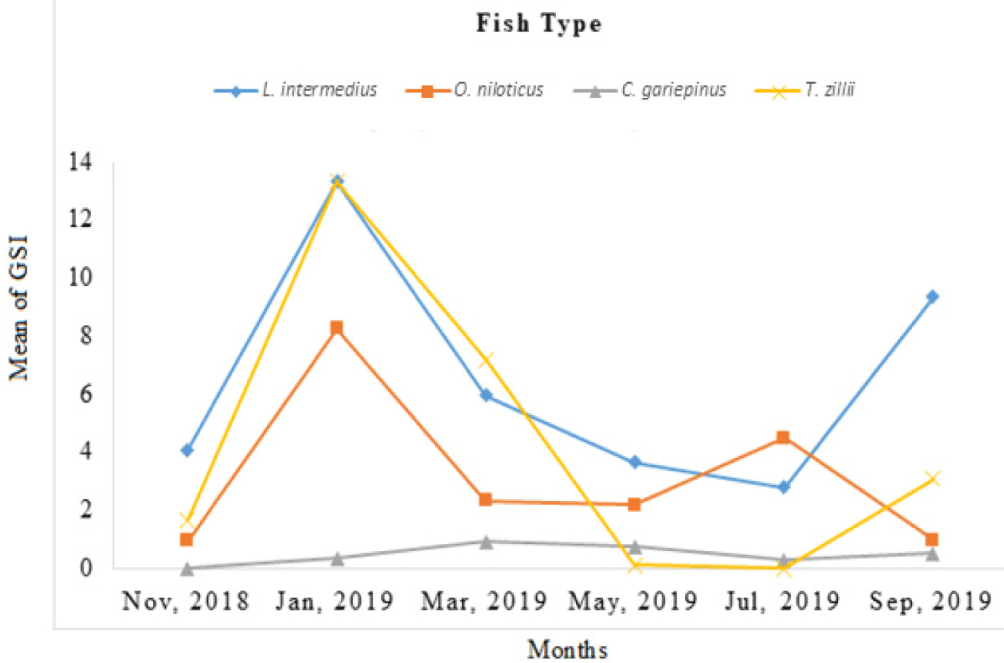
About 45 (69.23%) of the total captured ripe fish specimens were found in littoral habitat but 20 (30.77%) were found in the pelagic habitat of the reservoir. Generally, the littoral habitat (69.23%) was highly preferred breeding area for the studied fish species in the reservoir. The result showed a significant temporal variation of GSI for all fish species (ANOVA, p < 0.05), but no significant variation observed between the sexes (ANOVA, p > 0.05).
Monthly FCF values for the four fish species is presented in (Table 3). Monthly mean FCF values of T. zillii is 0.78 ± 0.0 in May 2019 (minimum) and 1.96 ± 0.05 in November 2018 (maximum). Maximum and minimum mean FCF for L. intermedius was 1.44 ± 0.23 in May 2019 and 0.87 ± 0.05 in Jul 2019, respectively. FCF value of O. niloticus (1.38 ± 1.15) was recorded in March 2019. The FCF value of L. intermedius, O. niloticus, C. gariepinus and T. zillii were temporally highly significant (p < 0.001) at 95% (Table 5).
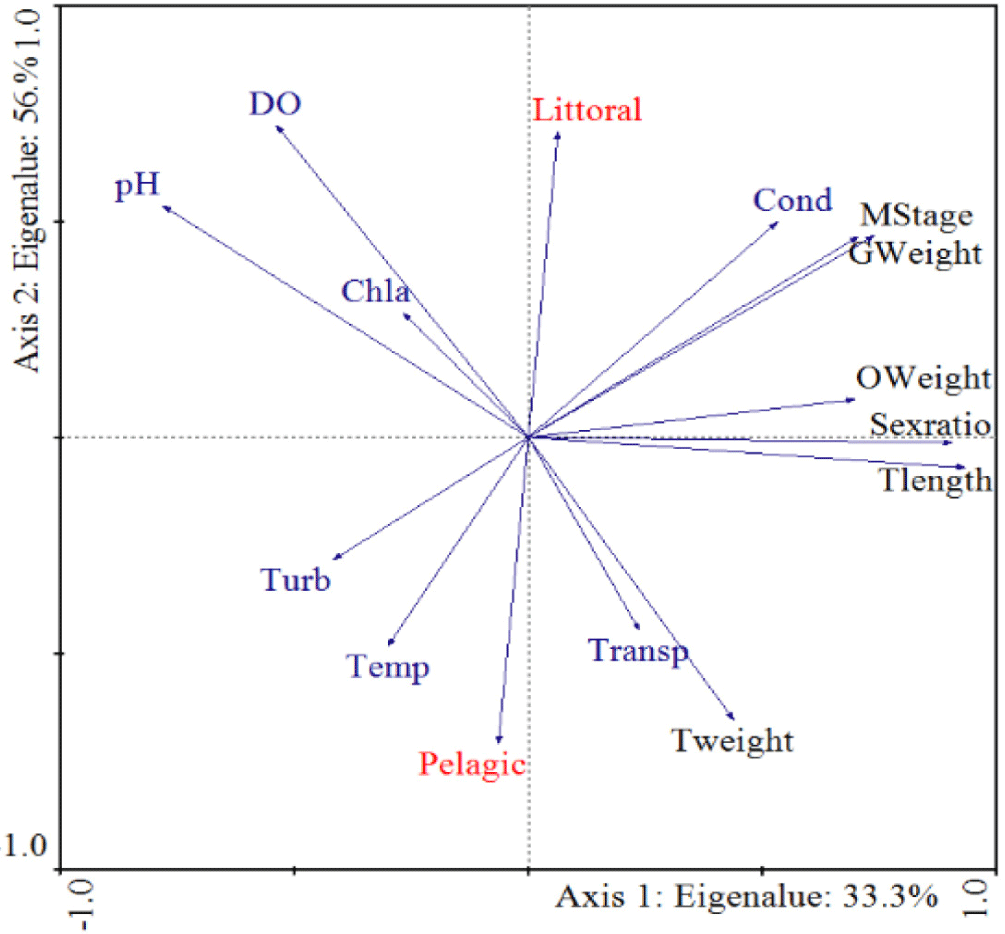
The relationships between some of reproductive aspects and various key physicochemical water quality variables in Mihtsab-Azmati reservoir was analyzed using a principal component analysis (PCA; Fig 6). The first and second axes of the PCA graph together explained 89.3% of the cumulative percentage variance of the relation. Axis 1 of PCA correlated positively and strongly with littoral site of the reservoir. Among the different water quality variables, both water transparency and conductivity were shown positive correlation with TL, TW, sex ratio, gonad weight, ovary weight and maturity stage. However, the other water quality variables such as pH, turbidity, temperature, dissolve oxygen and chlorophyll a were shown negative correlation with reproductive biology parameters.
Discussion
Water quality assessment is crucial for understanding the health of aquatic ecosystems and the factors that influence fish populations. By analyzing various water parameters, it is vital to evaluate the stability of the reservoir for fish growth, reproduction and survival (Rameshkumar et al., 2019). Mean value of dissolved oxygen, pH and turbidity were higher in the present findings compared with previous studies from similar reservoirs in Tigray (Dejenie et al., 2015). This might be due to high temperature record due to altitudinal variation and high rate of decomposition. The mean water transparency recorded in the present study was 190.68±3.59 cm which was higher than the values in Korir and Laelay Wukro dams in Tigray (28.17 and 29.55 cm, respectively) reported by Zebib & Teame (2017). This might be due to the catchment vegetation cover and absence of agricultural farmland nearby Mihtsab-Azmati reservoir.
With some exceptions males were slightly numerous than females during the present study. This is consistent with research conducted at Ethiopia’s Ribb Reservoir (Asres et al., 2023). Male dominance may arise from males migrating from spawning grounds to locations where, when egg fertilization is established, they are caught. While the female’s most likely move toward the flooded foliage and other regions surrounding the reservoir in order to continue protecting their young and avoiding predators. However, Teame et al. (2018) was reported 845 (46.28%) males and 981 (53.72%) females in Tekeze reservoir which disagrees with the present finding. There are a few possible causes for this, including behavioral differences between the sexes, sexual segregation during spawning, and fishing location. This fluctuation in the sex ratio may also result from males migrating from spawning sites to feeding areas in the shallow portion of the lake (where they are captured) once egg fertilization is complete. In order to safeguard the young and carry out the incubation process, the females may go near rocky and submerged vegetation, as well as away from potential predators like fishermen.
The relationship between TL and TW of the selected fish species were curvilinear, and as a result the line fitted to the data was described by the regression equation. The values obtained for the combined sex ratio of length-weight relationship showed that L. intermedius (b = 2.806), O. niloticus (b = 2.753), C. gariepinus (b = 1.973) and T. zillii (b = 2.526) which had negative allometric in their growth but only female T. zillii (b = 3.806) had positive allometric that is, the fish grows faster in weight than in length. The current finding is in line with negative allometric growth of O. niloticus in Koka Reservoir (Engdaw et al., 2013), Gilgel Gibe Reservoir (Wakjira, 2013); Ribb reservoir (Asres et al., 2023) all in Ethiopia. The nutritional sufficiency of the diet, environmental toxicity, and sexual maturity stage all have an impact on slope ‘b’ (Bagenal & Braun, 1978). Season, sex, length of captured specimens, population density, sexual maturity, age, habitat, stomach fullness, food quality or quantity, fish health, or environmental circumstances are some of the variables that may have an impact on the length-weight relationship’s parameters (Abera et al., 2015). Fish development rate can also be influenced by environmental variables as salinity, photoperiod, dissolved oxygen content, and water temperature (Zdanowski et al., 2001).
L50% is the minimum size at which the 50% population reaches maturity. Fish caught after reaching an L50% are therefore crucial to the sustainability of fisheries. In order to maintain the fish population, it is advised that the size at harvest be more than the size at initial maturity (Tefera et al., 2019). The sustainability of fish resources in bodies of water can be determined by measuring length at first harvest and L50%. Our finding suggested that, females of O. niloticus matured at a smaller size than males which is in line with previous finding in Ribb reservoir (Asres et al., 2023), and in Tekeze reservoir (Gebru, 2020). For L. intermedius, the L50% values for males and females were 24.5 cm and 18.75 cm, respectively. The females showed somewhat maturation at a smaller size than the males which is in contrast with the finding of Asres et al. (2023). Fish that mature at lower sizes tend to be those that live in harsh environment, are subject to fishing pressure, and have less food options available to them (Kebede et al., 2018). According to Senait (2015), fish raised in unfavorable settings reach a smaller adult size than those raised in favorable conditions. The reason for these variations is that sexual maturity is a function of size and can be impacted by several environmental conditions like as temperature, photoperiod, food availability and abundance throughout the year, as well as by geography.
As the current result indicated that for all the fish species in the study area the maturity stages (IV and V) were very few and we couldn’t observe for C. gariepinus and T. zillii. Pervious finding by Nyakuni (2009) in Albert Nile, Nebbi district was recorded with smallest ripe female O. niloticus of stage (IV) was 17.6 cm TL and 108 g body weight while the smallest ripe male was 17.8 cm TL and 200 g body weight. So, this finding is disagreeing with our current findings. Since females invest more energy on reproduction than males, they grow slowly and mature at relatively smaller sizes than males. Moreover, changes in Lake water level and associated factors, poor condition or overfishing (Lowe-McConnell, 1987) lead to a dramatic decrease in size at 50 % maturity. Tesfahun (2018) reported that the smallest female fish of the same species found with ripe gonads was 25.6 cm FL and weighed 162.2 g while the smallest male in breeding condition was 25.0 cm FL and weighed 205.3 g. But this disagrees with current finding because both male and female L. intermedius species appeared to attain sexual maturity at smaller size than the previous report.
The ratio of the gonadal weight to the total body weight is known as the gonadosomatic index (Gebremedhin et al., 2014). The GSI mean value for the male and female O. niloticus in the current study is 3.24 and 1.16, respectively. Our result seems to be slightly higher than the previous finding reported in Golinga reservoir in Ghana (Abarike & Ampofo-Yeboah, 2016). Temperature-related changes in metabolism and food availability lead to an increase in GSI. In the current study, the maximum male and female GSI of O. niloticus were found in January and November, respectively. So based on the results of GSI, it is possible to suggest that the fish has a biannual reproduction cycle. Similar findings also reported in Tekeze reservoir female O. niloticus observed two peaks of GSI values during February and August (Teame et al., 2018). However, our finding is disagreeing with other findings for the same species by Akongyuure (2020) in Tono reservoir, Ghana. GSI of L. intermedius was recorded (GSI = 8.038) in January and (8.554) in September for male and female, respectively in the study area. According to Kebede & Gubale (2016)L. intermedius had GSI mean value (3.44 ± 1.09) for females, and (3.69 ± 1.07) for males. But this disagrees with the current finding for the same species. However, all of the fish species have diverse intensive breeding time in the year, which mainly influenced by the accessibility by food and water temperature.
Fishery scientists have utilized the FCF index as a gauge of the population’s overall “fitness” or “well-being” (Jones et al., 1999). In the present study, the mean FCF of O. niloticus combined sexes in the study area were 1.602 which is similar to previous records in the Gilgel Gibe reservoir (Wakjira, 2013) but to some extent higher than the previous finding in Tekeze reservoir by Gebru (2020). Thus, feeding intensity of O. niloticus may be reduced if the fish actively engaged in breeding intensity activities. This could be the reason why the condition factor was relatively low compared with other previous findings in different water bodies. Shortage of food during dry season and starvation due to breeding behavior (mouth brooding) in O. niloticus during breeding season might reduce condition factor. Mean FCF of T. zillii in Lake Ziway was 2.08 for females, 2.05 for males and 2.06 for the population in general which was reported by Negassa & Getahun (2003). However, this disagrees with the present study because the mean value of FCF was 1.775 thus lower than Lake Ziway. This might be due to shortage of quality and quantity food. This study’s combined female mean Fulton body condition result for L. intermedius (1.058) is lower than the results reported in Gilgel Gibe I reservoir (1.18) (Wakjira, 2013). Compared to the aforementioned water bodies, findings suggested that the L. intermidius in this reservoir was in poorer physical condition. This might be because Mihtsab-Azmati reservoir was newly established young reservoir and continuously used for agricultural irrigation activities. The larger mean value of the body condition factor describes the higher energy content, higher food accessibility, reproductive potential, and favorable environmental situations for fish species. Factors like food availability or spawning activity influence FCF values of fish. This might be when food resources are plentiful, fish may exhibit higher feeding rates, leading to increased FCR values. This is because more of the ingested food is converted into growth. Furthermore, during spawning, fish often expend significant energy for reproductive activities, such as producing eggs or sperm and engaging in courtship behaviors. This can divert energy away from growth, resulting in temporarily lower FCR values (Kebede et al., 2018).
Conclusion and recommendation
The chi-square test analysis showed that sex-ratio (female:male) were deviated from the expected 1:1 in favor of males. The present finding of fish maturity stage was decreasing started from stage I (Immature) to stage V (spent) in all the identified selected fish species and therefore when fishing is intensified in this reservoir, catches are dominated by immature individuals. During data collection we couldn’t get enough data to analyze fecundity and were lower compared with other water bodies in Ethiopia, which could be indicative for rigorous fishing activity during breeding season and breeding area by the fishery cooperatives. The relationship between TL and TW of the selected fish species were curvilinear and the “b” values of both female and male of the selected fish species exhibits negative allometric growth as the value of “b” was less than 3. In Mihtsab-Azmati reservoir most of the fish species have their own peak spawning seasons once or twice in a year. Finally, different fishery management plans such as prohibiting fishing breading season, gear restrictions, mesh size regulations is needed in the reservoir for sustainable utilization of fishery resources.
