Introduction
Lakes are freshwater resources that have ecological significance for biodiversity and as life support systems for their surrounding areas (Heino et al., 2020). Lake Limboto, a tectonic lake located in Gorontalo, serves multiple ecological and economic roles. The lake is a regulator of hydrological functions, in particular with respect to flood control, surface water management, and the provision of potable water for many human communities (Lukman et al., 2017; Subehi et al., 2016). The rivers in the Lake Limboto watershed have potential for the generation of hydroelectric energy (Salim, 2021), while the lake and rivers have also been used for transportation (Akbar et al., 2022; Baga et al., 2024). Limboto Lake and its watershed provide habitat for many aquatic plants and animals, including several taxa native to Gorontalo (Lukman et al., 2017).
The living aquatic resources support the livelihoods of the local fishing communities through capture fisheries (using various gears such as gillnets, traps, hooks, etc.) and aquaculture (Akbar et al., 2022; Azizi et al., 1995; Krismono & Kartamihardja, 2010). According to (Lukman et al., 2017), the fish stocks in Lake Limboto can be classified into two groups: native lake fish and introduced fish. Native fish include gobies with the local names “payangga” or “payangka” (Giuris sp.) and “manggabai” (Glossogobius sp.) (Sukamto, 2016; Satria & Kartamihardja, 2017; Suryandari & Krismono, 2017). Fish introduced for aquaculture and/or released to increase fisheries production include carp (Cyprinus carpio) and tilapia (Oreochromis nilotica and Oreochromis mozambicus) (Ali et al., 2013; Purnama et al., 2023; Weimin et al., 2010), while the grass carp (Ctenopharyngodon idella) was introduced to control the invasive alien aquatic plant water hyacinth (Pontederia crassipes, formerly Eichhornia crassipes) in the lake (Krismono et al., 2010a, 2010b).
Environmental issues in Limboto Lake include sedimentation and a loss of extent and depth due to erosion in the watershed caused by land-use change such as deforestation and unsustainable agriculture practices, organic pollution from agriculture and sewage, and the introduction of alien invasive species of flora and fauna (Eraku & Permana, 2020; Haryono, 2004; Krismono et al., 2009, 2010a; Lukman et al., 2017). Over the past two decades, several surveys and studies report the dominance of introduced species. In 2006, a survey of fish biodiversity in Limboto Lake identified 11 fish from 6 families, of which only four species (two gobies, an anguillid eel and a mullet) can be considered native (Suryandari & Krismono, 2008). Most of the non-native fish seem to have been introduced to the lake in the first half of the 20th century (1914−1947); however, the Nile tilapia (Oreochromis niloticus) and the common carp (C. carpio) are thought to have been introduced in the late 1800’s (Haryono, 2004). These threats have led to a loss of native submerged aquatic vegetation and native biodiversity, in particular native fish, as well as a decrease in fisheries productivity (Krismono et al., 2010a; Lukman, 2010; Lukman et al., 2017).
Recognising the importance of this lake, Limboto was declared one of 15 priority lakes in Indonesia in 2010 (Lukman et al., 2017), a designation re-affirmed in 2021 under Presidential Regulation 60/2021 (Republic of Indonesia, 2021). One of the key issues to be considered in the management of Lake Limboto is the decline in the populations of native fisheries species which have traditionally been important for their economic value and contribution to food security. These include previously abundant fish such as two gudgeons (Giuris spp.), tank goby (Glossogobius sp.) and striped snakehead Channa striata, as well as more rarely encountered fish including mullets (Planiliza sp.) and fishes only identified by their locals name (Auliyah, 2019; Suryandari & Krismono, 2008).
Considering the important role of Lake Limboto for local communities and the Gorontalo region (Melo et al., 2024), sustainable management efforts are needed, in particular strategic conservation action to support the priority lakes program in Lake Limboto. The formulation of a concept to restore the population of native fish species is needed to maintain biodiversity and the benefits gained from the fisheries sector (Ormerod, 2003). Time-series data are vital for natural resources management, in the fields of conservation and fisheries (Collen et al., 2009; Charles, 2023; Froese et al., 2019; Pitcher, 2001), in particular historical data on species catch composition based on the type of fishing gear used. This study presents catch composition data from the gillnet fishery in Lake Limboto with an emphasis on native fish fauna biodiversity and conservation.
Materials and Methods
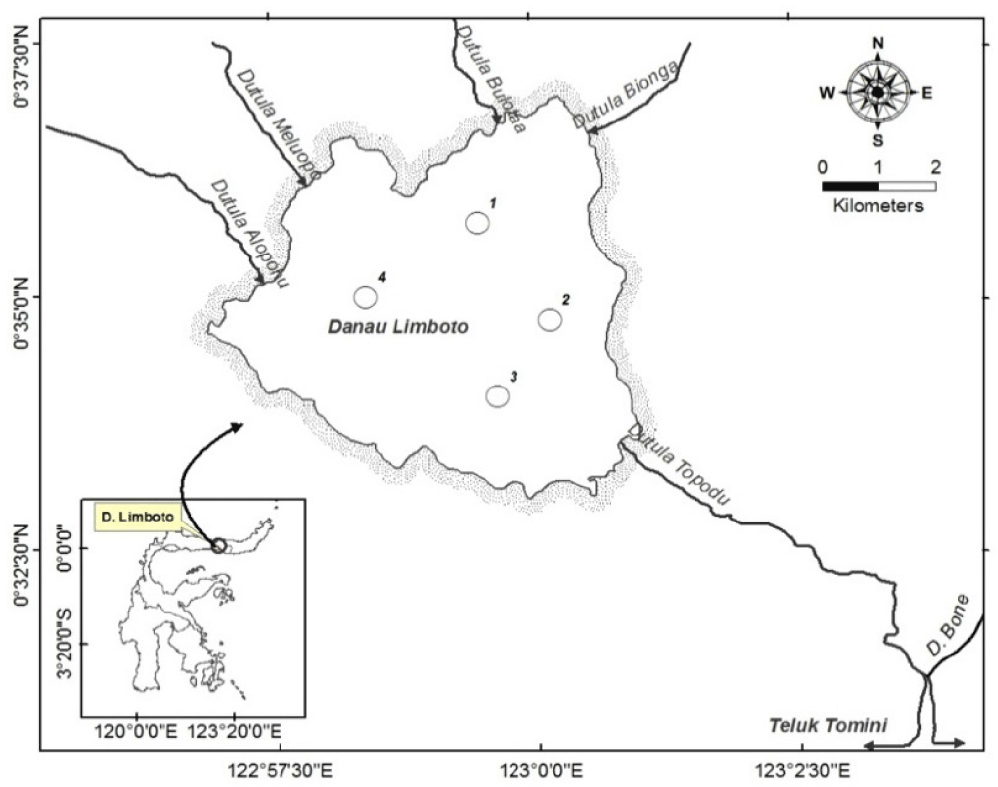
Data were collected in Lake Limboto from mid-February to early April 2011. Four sampling stations (North, East, South, and West) were selected to represent areas of the lake with different bio-ecological characteristics. The sampling station locations are shown in Fig. 1.
The materials used in this study included fish samples collected over a period of 5 weeks using gillnets with the local name “buili”. The samples fixed in 4% Formalin solution and then preserved in 80% alcohol. The equipment used included buili gillnets (mesh size 2.25 in, length ≥ 100 m, and depth 2−3 m), a fish ruler (precision 1 mm), digital scales (precision 0.01 g), plastic bags, jars, buckets, labels, tweezers, stationery, a camera, and identification books.
Specimens were collected by fishermen using buili gillnets, with a total of 32 fishing trips to provide a representative sample of the population. Fish were caught at each observation station. The sampling sequence was: Station I, Station II, Station III, and Station IV. Each station was sampled 8 times. The nets were operated passively; after the nets were deployed in the water in the morning, the boat and gear were allowed to drift, typically for 2−3 hours. All caught fish were collected if the catch was less than 100 individuals; if the catch exceeded 100 individuals, only 100 were taken. The catch at each station was documented (photographed) and the following data were recorded for each fish specimen: species, total length, and weight. Additional observations made during the study period included a visual assessment of aquatic vegetation at each station.
The catch data obtained during the study were compiled and tabulated in Microsoft Excel 365. The data were analysed descriptively to determine the catch composition, the number of individuals and size distribution for each species. These data were compared with historical data from other studies on the species present in Limboto Lake and their relative abundance over several decades.
Results
The gillnet catch data over the study period (Table 1) show that the most frequently captured fish species were all introduced (alien) fishes: tilapia (O. niloticus, and Oreochromis mossambicus), Java barb (Barbonymus gonionotus), snakeskin gourami (Trichopodus pectoralis), and common carp (C. carpio). Species caught in small numbers included the native tank goby (Glossogobius sp.), mullet Planiliza sp. and spotted scat (Scatophagus argus), as well as two fish with ambiguous status, the climbing perch (Anabas testudineus) and striped snakehead (C. striata).
The fish caught using gillnets were mostly relatively small compared to reported values for the mean length at first maturity for their respective species (Table 2). The mean total length and weight were around 10 cm and 38 g, respectively. The native tank goby (Glossogobius sp.) ranged in length from around 12 to 20 cm (Fig. 2).
| No. | Species | n | Length (cm)1) | Weight (g)1) | Length at first maturity | |
|---|---|---|---|---|---|---|
| Lm (cm) | Reference | |||||
| 1 | Anabas testudineus | 19 | 8 – 12.1 (10.05) | 16.34 – 78.22 (47.28 | 8.4 | Khatun et al., (2019) |
| 2 | Barbonymus gonionotus | 268 | 7.6 – 17.1 (12.35) | 5.8 – 120.18 (62.99) | 12 – 19 | Hossain et al., (2016) |
| 3 | Channa striata | 1 | 24.5 – 24.5 (24.5) | 160.63 – 160.63 (160.63) | 18 | Froese & Pauly, (2024b) |
| 4 | Cyprinus carpio | 8 | 12.4 – 15.1 (13.75) | 40.26 – 89.21 (64.73) | 18 – 40 | Hossain et al., (2016) |
| 5 | Glossogobius sp. | 13 | 7.6 – 15.5 (11.55) | 5.8 – 46.76 (26.28) | 8 – 12 | Dinh et al., (2021) |
| 6 | Liza sp. / Planiliza sp. | 2 | 11 – 11 (11) | 24.35 – 24.35 (24.35) | ND | |
| 7 | Oreochromis mossambicus | 650 | 7.3 – 17 (12.15) | 15.64 – 147.63 (81.64) | 14.4 | FishBase |
| 8 | Oreochromis niloticus | 976 | 6.5 – 16.9 (9.9) | 9.9 – 119.04 (64.47) | 15 – 32 | Hossain et al., (2016) |
| 9 | Scatophagus argus | 1 | 7.9 – 7.9 (7.9) | 24.88 – 24.88 (24.88) | 11 – 15 | Dinh et al., (2021) |
| 10 | Trichogaster pectoralis | 11 | 7.0 – 12.4 (9.1) | 10.47 – 74.63 (42.55) | 18 | Amornsakun et al., (2004) |

The 25 taxa reported from Limboto Lake between 1994 and 2019 include indicate changes in fish biodiversity over time (Table 3). The data from several studies also indicate changes in relative abundance based on catch composition, with an increasing trend for most introduced species and a decreasing trend in native species catch (Table 4).
Data from Krismono et al. (2018).
Data from Suryandari & Krismono (2008).
Data from Suryandari & Krismono (2008).
Discussion
The catch composition varied between sampling stations in terms of fish species, size, and number of individuals. Of the ten fish species identified, the introduced Nile tilapia (O. niloticus), Mozambique tilapia (O. mossambicus), Java barb (B. gonionotus), snakeskin gourami (T. pectoralis), and common carp (C. carpio) accounted for between 96.5% and 99.1% of the catch at each station, and 98.7% of all fish caught during the study period. Collectively, the native fish and those with ambiguous (but most likely long-established introduced) status accounted for just 1.3% of the total catch, reflecting a decline in the native fish population of Limboto Lake (Table 3), including the once-abundant and locally important Gobiiformes fisheries species, in particular Glossogobius sp. (Hasim et al., 2021; Suryandari & Krismono, 2017).
Alien species are defined in the Scientists’ warning on invasive alien species (Pyšek et al., 2020) as “those whose presence in a region is attributable to human actions, deliberate or inadvertent, that enabled them to overcome biogeographical barriers,” and can become invasive when they breed successfully and form self-replenishing populations. Traits such as general hardiness, ease of breeding, rapid growth rates and the ability to efficiently utilise a wide range of food sources, including organic waste, have helped make tilapia (genus Oreochromis) popular as aquaculture species, but have also enabled them to survive in the wild in many habitats worldwide, and to dominate the fish fauna in many water bodies far from their natural distribution in Africa after intentional or accidental release (Hutchison et al., 2011). The Mozambique tilapia O. mossambicus had been introduced to at least 89 countries by 2006 and is rated as one of the 100 most invasive species in the world (GISD, 2024a). Ironically, this tilapia is considered vulnerable to extinction in its native habitat, mainly due to introduction of and subsequent hybridisation with, the Nile tilapia O. niloticus (Russell, 2012). The Nile tilapia O. niloticus is also considered highly invasive, with especial concern regarding brackish- and salt-water-tolerant strains and hybrids which have been developed for aquaculture (GISD, 2024b), and assessed as least concern (LC) in the International Union for Conservation of Nature (IUCN) Red List, despite threats to the native population in eastern Africa from hybridisation and localised overfishing (Diallo et al., 2023). Surprisingly, Indonesia is not listed as a country with established introduced or feral populations in the Global Invasive Species Database (GISD) profile for the Nile tilapia, despite extensive reference to the Genetically Improved Farmed Tilapia (GIFT) project and the resultant strain, which is exceptionally invasive and has been widely introduced in Indonesia (Subasinghe et al., 2021), and the inclusion of Indonesia in the list of countries with introduced populations in the IUCN Red List assessment of O. niloticus (Diallo et al., 2023). This indicates a need for updating the GISD profile of O. niloticus. Limboto Lake is one of many waterbodies where Nile and/or Mozambique tilapia have become established in the wild in Indonesia (Herjayanto et al., 2019; Robin et al., 2023). Tilapia have been implicated in the decline of native species, including endemic fish and invertebrates in many countries, including Sri Lanka (Pet & Piet, 1993), the Philippines (Cuvin-Aralar, 2016), and Indonesia (Robin et al., 2023). In addition to their high capacity for adaptation, the fast reproduction rate is a key factor enabling tilapia to out-compete many other fish species, in particular the fish native to many habitats (Cuvin-Aralar, 2016; Herjayanto et al., 2019). Parasites and diseases co-introduced with these (and other) alien species (Ali et al., 2013) may also have played a role, as in other Sulawesian lakes (Herder et al., 2012, 2022).
The overall catch volume and the number of tilapia caught were highest at Station I, followed by Station IV. The samples collected from stations I and IV respectively comprised 40% and 10% of all O. niloticus caught during the study and 41% and 23% of all O. mossambicus. The comparatively high overall catch volume at these two stations was likely influenced by their closeness to major rivers flowing into Lake Limboto and leading to elevated nutrient content and primary productivity in the nearby areas of the lake, and in comparatively deep waters. Stations II and III were closer to the outlet, in shallower waters (around 1.5–2.5 m depth). Station III was nearest to the outlet in an area with no canopy-forming plants and used for floating net cage aquaculture. In other lakes, similar fish cages have caused a decline in water quality (Hutajulu & Harahap, 2023), and this could be one reason for the lack of vegetation and comparatively low catch at this station.
At Station IV, one visible sign of increased primary productivity was the high (around 40%) coverage of the lake surface with aquatic plants, mainly the water hyacinth P. crassipes which had become well established and was seen forming often dense mats in several areas of the lake during the study. In several areas the P. crassipes-dominated vegetation was so dense that it made access difficult for fishing boats and interfered with fishing activities. Populations of this alien invasive species tend to fluctuate (Cuvin-Aralar, 2016), but can expand extremely fast through sexual and asexual propagation and are extremely expensive and hard or even impossible to eradicate once established (Degaga, 2019; Dasgupta, 2021). However, although it has caused catastrophic declines in some lake fisheries (Degaga, 2019; Pyšek et al., 2020), the water hyacinth can provide habitat, in particular a refuge from predation and feeding ground for some aquatic animals, in particular invertebrates (Montiel-Martínez et al., 2015; Nguyen et al., 2015), and can be controlled to some extent through reducing nutrient levels (Lukman et al., 2017). This is likely one factor, as by 2006 the waters of Limboto Lake were generally in eutrophic or even hypereutrophic condition a consideration (Krismono et al., 2009).
While tilapia were the dominant taxa at all stations, species richness and species composition differed between the four sampling stations in this study. Eight of the eleven species were caught at Station I and Station IV, while six species were found at Station II and five at Station III. The only species other than the two tilapias to be caught at all four stations was the alien Java barb B. gonionotus, representing nearly 14% of the total catch and around 5−16% of the catch at each station. The remaining eight species were each caught at 1−3 of the four stations. Like the tilapia, this introduced species was caught in the greatest numbers at Station I followed by Station IV. Previously known as Puntius gonionotus, this fish is native to areas in Southeast Asia including eastern Indonesia (Java, Sumatra), Mekong and Chao Phraya basins, and the Malay Peninsula (Froese & Pauly, 2024a). Introduced to several countries, mainly under mosquito control and integrated rice-fish farming programs, the Java barb has had varied impacts (positive and negative) on aquatic ecosystems, and can be a carrier of diseases affecting native species, including snakeheads, gobies, and eels (US FWS, 2018). The Java barb is considered invasive and a threat to endemic and native lacustrine species in Sulawesi (Herder et al., 2022; Kottelat, 1990; Omar et al., 2020; Serdiati et al., 2021; Yanuarita et al., 2020), as well as in several other countries (Joshi, 2006; US FWS, 2018).
The anabantids A. testudineus and C. striata were once abundant in Limboto Lake. Both are increasingly valuable as food fish and for use in the pharmaceutical and nutraceutical industries (Ndobe et al., 2014, 2019). While they may have reached at least some waterbodies in Sulawesi without human intervention, and thus be native to the island, it is also possible that they may have been introduced to some of their present Sulawesian distribution in the very distant past (Ndobe et al., 2020). Although considered as local fish and woven into traditional culture in some areas (Ndobe et al., 2014, 2019), there is strong evidence for their introduced status in certain waterbodies, such as the Poso and Malili complex ancient lakes (Herder et al., 2012, 2022). Whatever their original mode of arrival and status, in Limboto Lake, as in several other lakes and wetlands, they long co-existed alongside other local fisheries species considered native such as the gobies of the genera Giuris and Glossogobius. Despite their IUCN Red List status of LC (Ahmad et al., 2019; Chaudhry et al., 2019), several populations are threatened by overfishing and/or the introduction of invasive species such as tilapia, barbs, and others (Ndobe et al., 2014, 2019, 2020).
The shift in fish community composition in Limboto Lake is reflected in the species richness and catch composition data from several studies over the period 1994 to 2019 (Table 3). Several years earlier, a variety of native fish species were found, but by the time of this study only tilapia and Java barb remained as viable fisheries species. Two surveys conducted in 2006 each recorded four native species. Both reported manggabai (recorded as Glossogobius giuris), and a freshwater eel (Anguilla sp.). One recorded the payangka (recorded as Ophiocara porocephala), a mullet (Mugil sp.) with the local name bulalao and seven introduced species, while the other identified two types of payangka (recorded as O. porocephala and Ophiocara sp.), and five introduced species (Krismono et al., 2018).
Several fish species found in the past were totally absent from the catches in this study. The identity of these species is uncertain, but the local names include mangaheto (a red-coloured fish), botua (a white scaleless fish), and boidelo (a grey fish). The 2010 fisheries production statistics for Limboto Lake (Gorontalo Provincial Government, 2010) show production volume heavily dominated by tilapia (66.5%); of the other five species named, only one was a native fish (Glossogobius sp.), comprising 10.6% of production volume, while the remaining four named introduced species collectively contribute 9.7% and a category comprising miscellaneous fish species contributed 13.2% (Table 4).
The length and weight data in Table 2 show that the fish caught in Limboto Lake using gillnets were mostly relatively small. This may be related to the sampling station, as small fish tend to inhabit where they can find suitable food and/or shelter, such as shallow waters near river inlets and areas with aquatic vegetation. Specimens of three species now rarely caught (A. testudineus, C. striata, Glossogobius sp.) were mostly above published data on length at first maturity (Lm), and therefore likely adult, while the majority of specimens of the other seven species were close to or below Lm (Table 2). For diadromous species such as the amphidromous S. argus and catadromous Planiliza sp., low numbers of juvenile individuals may represent infrequent recruitment through the outlet, from a resident breeding population or other nearby populations. These considerations could also apply to the catadromous freshwater eels Anguilla spp, including Anabas bicolor (Krismono et al., 2018) and Anabas celebensis. In addition to conditions within the lake and potential sampling bias, low numbers or loss of these and other diadromous species could be related to conditions in the coastal waters of Tomini Bay and in the outlet connecting Limboto Lake to the bay.
For the fisheries species, the low mean and maximum sizes could be related to factors such as seasonality and food availability, size/age habitat segregation, recent release of young fish, or could reflect low values of Lm for the feral Limboto Lake populations, and the catch size distribution data can be used as baseline to inform fisheries resource management in Lake Limboto. The gillnet fishing gear used is size-selective, so that catch efficiency depends on the size of the captured fish. The mesh size could be adjusted to obtain a higher proportion of larger fish, and possibly target suitable species in a specific fishing area, to achieve a better species balance and support the longer-term sustainability of lake fisheries.
The distribution and condition of fish stocks within the lake are important for fisheries resource management in Lake Limboto. In this respect, the catch distribution across the sampling sites provides information of value for management, especially if incorporated as part of a time series together with more recent or future data sets. For example, such data can be of use in spatial planning (e.g., zonation) and law enforcement, and could inform regional regulations on resource management in Lake Limboto, including the operation of environmentally friendly fishing gear and fisher-oriented human capacity building.
Data on the species composition at a particular point in time can providing a broadly accurate picture of biodiversity, and can serve as important benchmarks in ecological studies; however, accurate identification is extremely important in evaluating biodiversity, managing fisheries, and analysing population dynamics. Taxonomy and the changes in scientific names can pose a considerable challenge in seeking and analysing historical data on fish biodiversity, as evolutionary relationships are refined and redefined, a process which has accelerated with the advent of molecular biology methods, in particular the use of deoxyribo nucleic acid (DNA) sequences in phylogeny. Meanwhile, cryptic species and certain life stages can also pose a challenge for identifying specimens based on external morphology. Several species identified during this study have undergone changes in taxonomic nomenclature, and uptake of revised names at genus and family level has often been delayed and/or patchy, resulting in several (now valid or invalid) synonyms being used in relevant literature and data sets for some species. The genus name Liza for the mullets found around Sulawesi has been changed to Planiliza, while identification to species level based on morphological traits is highly prone to error in this genus (Durand et al., 2017). Obsolete synonyms are still commonly used for the striped snakehead C. striata (formerly Ophiocephalus striatus), the Java barb B. gonionotus (formerly P. gonionotus), and the snakeskin gourami T. pectoralis (formerly Trichogaster pectoralis). Meanwhile, tank goby taxa including G. Giuris and G. aureus are now considered as species complexes comprising several species-level taxa, with mismatches between morphological and molecular identification and indications of widespread misidentification (Abdulmalik-Labe et al., 2022; Hoese & Hammer, 2021; Hammer et al., 2021; Truong et al., 2022).
One of the native fish not found in this study was the eleotrid goby locally called payangka (Giuris sp.). This fish was considered abundant up to the beginning of the 21st Century (Haryono, 2004). Originally described as Eleotris aporos Bleeker 1854 and reported in several studies on the fish fauna of the lake as Ophieleotris aporos (Haryono, 2004; Hasim et al., 2021; Lukman, 2010), the true identity of the eleotrid goby in Limboto Lake is open to question. The taxonomy of the Eleotridae, and in particular the genus Giuris, has undergone several revisions. Many named species were synonymised as Giuris margaraticeus; however, more recent studies have determined that this taxon represents a species complex comprising at least eight species (Keith & Mennesson, 2020; Keith et al., 2020). The payangka in the nearby Bolano Sau Lake in Central Sulawesi has been determined as Giuris laglaizei Sauvage, 1880 (Ndobe et al., 2023), while three other Giuris species (Giuris tolsoni [Bleeker 1854]; Giuris viator, [Keith, Monnesson, Lord, Hubert 2020]; and Giuris margaritaceus [Valenciennes 1837]) have been identified from Sulawesi water bodies (Keith et al., 2020). The Giuris sp. know locale as payangka in Tondano Lake (Pangemanan et al., 2020) is thought to have been introduced from Limboto Lake and they both differs genetically from all other Sulawesian Giuris populations studied (Ndobe et al., 2023) as does the Giuris sp. known locally as hulu’u from Limboto Lake (Lamadi et al., 2023; Ndobe et al., 2023). Lack of evidence of presence is not proof of absence, especially in situations (such as that in Lake Limboto) where monitoring and exploration of aquatic biodiversity has been limited in scope and sporadic (Herder et al., 2022).
Molecular methods based on short nucleotide sequences that are largely conserved within species but differ more between species and higher taxonomic levels are increasingly used; in particular DNA barcoding using mitochondrial DNA (mtDNA), especially segments of the cytochrome oxidase I (COI) gene, can play a key role in accurately identifying species, including freshwater fishes (Bhattacharya et al., 2016; Hebert et al., 2003; Hubert et al., 2015; Ward et al., 2009). This technique is particularly useful for recognizing individuals at various stages of life, incomplete specimens, and cryptic species (Chakraborty & Ghosh, 2014; Durand et al., 2017; Ndobe et al., 2023). With the aid of reliable vouchered reference sequences (Becker et al., 2011), which are currently lacking in public databases for some taxa in the genera Giuris and Glossogobius, such methods should be capable of resolving the uncertainty regarding the identity of the Giuris sp. and Glossogobius sp. in Limboto Lake.
In conclusion, fishermen using gillnets caught a total of 10 fish species over the study period, with only small numbers of native fish. The dominant fish species were all alien introduced fish: Nile tilapia (O. niloticus), Mozambique tilapia (O. mossambicus), and Java barb (B. gonionotus). However, the method used and sampling pattern may have missed some species present, for example if they were in other parts of the lake, too small or otherwise not easily caught by the gillnet fishing gear used. The changes over time demonstrate a need for monitoring, in particular to assess the effectiveness of interventions to improve the management of this priority lake. Furthermore, this study highlights a need for resolving taxonomic uncertainties regarding the species present in Limboto Lake, in particular the native goby species. In addition to classical survey and identification methods, including length-frequency monitoring of key species of fisheries and/or conservation concern, the use of molecular methods such as DNA barcoding and eDNA could resolve the taxonomic uncertainties and provide a more complete picture of the aquatic community and fish biodiversity to inform fisheries and environmental management programs as well as biodiversity conservation.
