Introduction
The regulation of feeding and energy metabolism in fish is an essential process for survival and development, allowing them to obtain necessary energy for growth, and maintenance of physiological functions. Moreover, feeding processes are influenced by external factors such as light, temperature, habitat, and salinity, as well as internal factors including developmental stage and genetic influences (Schreck & Tort, 2016; Volkoff & Rønnestad, 2020). In particular, light serves as an important external stimulus for the physiological activities of fish, stimulating the hypothalamic-pituitary-adrenal (HPA) axis, which directly influences feeding behavior through increased secretion of appetite-related peptides (Canosa & Bertucci, 2023; Falcón et al., 2010).
Light emitting diodes (LEDs) have garnered attention in the aquaculture sector as lighting strategy due to their long lifespan and low energy consumption. LEDs can effectively emit light at specific wavelengths, making them widely utilized in studies inducing physiological changes in fish, leading to the development of aquaculture methods that enhance growth efficiency and stress reduction (Güller et al., 2020; Luchiari & Freire, 2009; Villamizar et al., 2011; Zheng et al., 2024). However, studies investigating the impact of light spectra on the distribution of endocrine cells and growth in fish remain relatively scarce.
Feeding behavior in fish is regulated by various appetite-related hormones. Among these, ghrelin (GHRL) is an orexigenic hormone secreted from the stomach, primarily produced in the P/D1 (human) or X/A (rat and dog) equivalent cells of the gastric oxyntic mucosa (Date et al., 2000). GHRL secretion is stimulated by factors such as IGF-1 and leptin, promoting the release of growth hormone (Avau et al., 2013; Tannenbaum et al., 2003). Additionally, GHRL acts on the hypothalamus to stimulate appetite, decreasing after food intake (Cummings et al., 2001; Kojima et al., 1999; Tschöp et al., 2000). Neuropeptide Y (NPY) is a neurotransmitter primarily found in the brain, including peripheral tissues such as the hypothalamus, sympathetic ganglia, and gastrointestinal tract. Composed of 36 amino acids, NPY plays crucial regulatory roles in both the central and peripheral nervous systems, particularly in appetite regulation, energy balance, blood pressure, and gastrointestinal motility (Danger et al., 1990; Holzer et al., 2012). Pancreatic polypeptide (PP) is found in various vertebrates, including fish and mammals, and plays a critical role in regulating digestion and energy metabolism. It modulates the exocrine functions of the pancreas and is involved in appetite regulation and gastrointestinal motility (Zhu et al., 2023). Cholecystokinin (CCK) is a hormone secreted from the digestive tract that functions in inhibiting gastric secretion, regulating bile secretion, promoting digestive enzyme secretion, and appetite regulation (Gibbs et al., 1973; Rehfeld, 2017). In fish, CCK expression is known to decrease primarily during fasting periods, and its effects on various digestive processes are regulated by receptor activation (Furutani et al., 2013). Goblet cells are distributed in the mucosal epithelium of the digestive tract, where they secrete mucus to protect and lubricate the intestinal lining. Enteroendocrine cells, on the other hand, secrete hormones that regulate digestion and metabolism. These cells detect the components of ingested food and activate the gut-brain axis by releasing hormones such as CCK, NPY, and GHRL. Through this activation, they help regulate appetite, digestive enzyme secretion, and gastrointestinal motility (Dezfuli et al., 2017; Rombout, 1977).
Red seabream (Pagrus major) is a highly demanded species in East Asian countries; however, uncertainties in aquaculture have increased due to mortality from climate change and disease. While research on other areas such as temperature has been actively conducted (Biswas et al., 2006), studies focusing on light remain relatively insufficient. Therefore, this study compared the growth and regulation of appetite-related hormones in the digestive tract of red seabream under different LED spectra. This study would expand understanding of various light exposure to the digestive physiology and support aquaculture development of red seabream.
Materials and Methods
This study was approved by the Animal Experiment Ethics Committee of Pukyong National University and was conducted in strict compliance with animal welfare regulations (Approval Number: PKNUIACUC-2023-58).
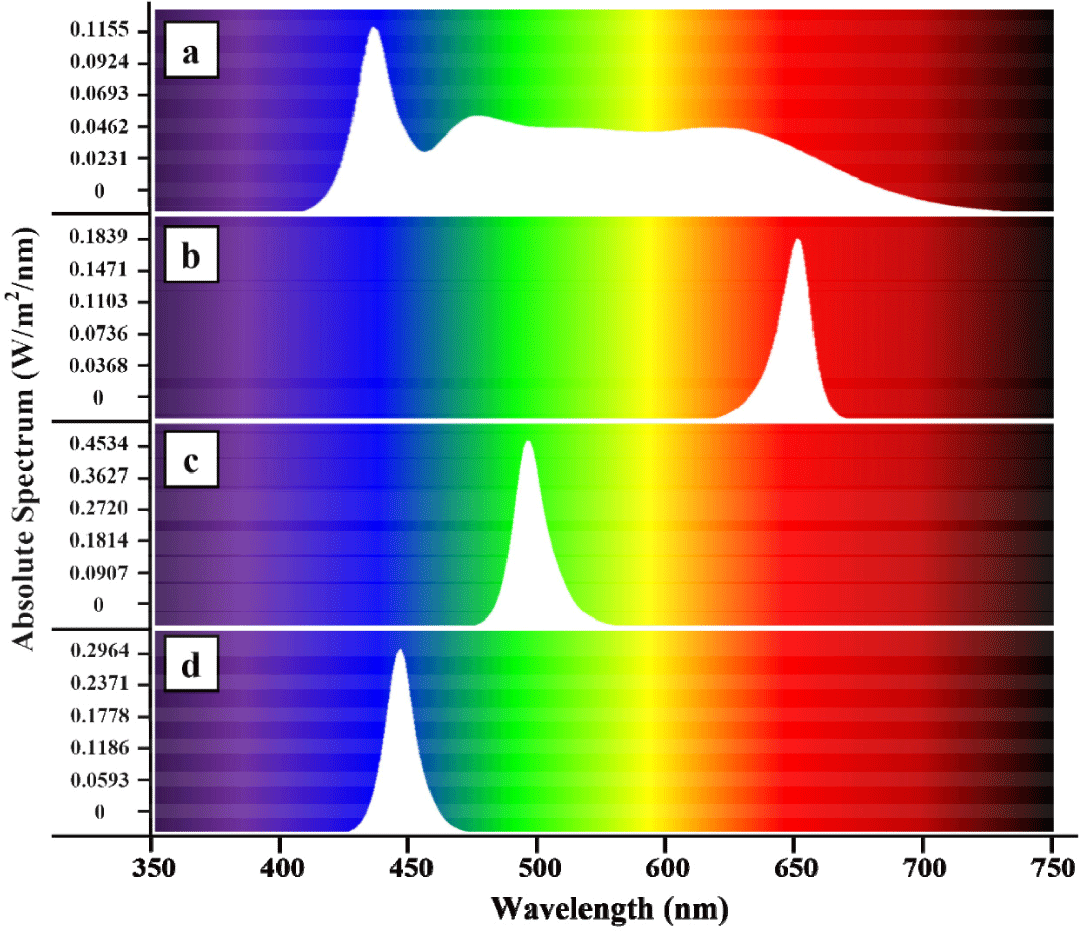
The rearing tanks were made from 350 L circular polypropylene tanks (40 × 40 × 90 cm) fitted with lids that housed LEDs emitting monochromatic light, effectively blocking incoming external light. LEDs were custom made to emit different light wavelengths-red (660 nm), green (518 nm), blue (450 nm), and white (full spectrum), allowing for the division of experimental groups (replicated thrice; Fig. 1; Kim et al., 2025). White-colored light served as a control treatment due to its high similarity to natural light. The LEDs were installed 50 cm above the water surface, programmed to turn on at 8 AM and off at 8 PM, establishing a photoperiod of 12L:12D, and brightness was set to 150 lux measured by a light meter (TES-1339, TES Electrical Electronic, Taipei, Taiwan).
The red seabream used in the experiment were supplied by the Gyeongnam Fisheries Resources Research Institute (Tongyeong, Korea) and transported to the facility of Institute of Fisheries Sciences, Pukyong National University (Busan, Korea). Fish underwent a two-week acclimatization period in a separate, no light treatment tanks before actual experiment commenced. Thereafter, 14 fish per tank (42 individuals per light treatment) were randomly distributed to tanks. The average weight of the red seabream used was 122.96 ± 19.12 g. Fish were fed a commercially formulated feed twice daily at 8:30 AM and 6:30 PM until apparent satiation, which was determined based on the lack of feeding response. The rearing water was supplied through a flow-through system and complete water exchange was conducted 18 times per day. Aeration was provided, and a temperature of 20.06 ± 0.29°C was maintained using heated seawater (NIFS, 2020). Fish were subjected to body measurements and collection of gastrointestinal samples. Fish were anaesthetized with 2-phenoxyethanol (100 ppm), and the total length (cm), standard length (cm), body height (cm), body width (mm) were measured using a vernier caliper (CD-20APX, Mitutoyo, Kawasaki, Japan). The weight of the liver, viscera and gonad were subsequently measured in an electronic scale (AJ-4200E-D, Shinko, Nagano, Japan). Consequently, using sterile dissecting tools, liver, stomach, and intestine were separated from the body, immediately frozen in liquid nitrogen and stored at −80°C until analysis. Samples intended for histological analysis were fixed in 10% neutral-buffered formalin.
For fluorescence in situ hybridization (FISH) analysis of NPY and GHRL, stomach and intestinal tissues were analyzed from five fish per experimental group. The fixed tissues were made into paraffin blocks and sectioned at 5 μm thickness, which were then mounted onto glass slides. Probes for NPY (5’- TTGTGGCGTAAGTCAACC -3’; GeneBank#: LC314445.1) and GHRL (5’- AACTTGGTGAGCTGGCTG -3’; GeneBank#: LC155430.1) were added and incubated overnight at 55°C. The slides were then washed with phosphate-buffered saline with Diethyl pyrocarbonate treated water and intensity of expression was observed within 2 min exposure time for all samples using a fluorescence microscope (Olympus IX51, Olympus, Tokyo, Japan).
For IHC analysis of PP and CCK, liver and intestinal tissues were collected from five fish per experimental group. The fixed tissues were made into paraffin blocks and sectioned at a thickness of 8 μm, which were then mounted on lysine-coated poly-prep slides (Sigma-Aldrich, St. Louis, MO, USA). Immunostaining was performed according to the protocol of the VECTASTAIN ABC Kit (Vector Lab, Newark, CA, USA), with CCK antibody (Santa Cruz, TX, USA) and PP antibody (Santa Cruz, SC, USA) diluted 1:500 in bovine serum albumin blocking buffer and incubated overnight at 4°C. The sections were subsequently developed using the DAB Substrate Kit (Vector Lab) and observed and photographed with an OLYMPUS SEX41 (Olympus).
For histological analysis, liver, stomach, and intestinal tissues were collected from ten fish per experimental group. The fixed tissues were made into paraffin blocks and sectioned at a thickness of 8 μm, which were then mounted on slides. The histological structures were confirmed using hematoxylin (Dako Products, Santa Clara, CA, USA) and eosin (Sigma-Aldrich) for staining. Alcian blue-periodic acid-schiff (AB-PAS) staining was performed to assess the development of goblet cells. The prepared slides were observed and photographed using an OLYMPUS SEX41 (Olympus). The number of goblet cells was measured in a randomly selected area of 1,000 μm² using Michrome 6 (Tucsen, Fuzhou, China) and Mosaic 2.1 (Tucsen).
Quantitative data were analyzed in SPSS ver. 22 (IBM, Armonk, NY, USA) using one-way analysis of variance (ANOVA) after passing normality and homogeneity assumptions. Significant difference was set at p < 0.05, and post-hoc test was done using Duncan’s multiple range test. Results are shown as mean ± SE.
Results
The effect of different LED spectrum on the growth performance of red seabream is shown in Table 1. Body weight and length morphometries (total length, standard length, body height, body width) were statistically affected by LED light treatments (p < 0.05). Fastest growth was observed in blue followed by green and white light treatments. Red light induced the slowest growth among light treatments. However, liver weight, visceral weight, and gonad weight was not statistically influenced by any LED treatments (p > 0.05).
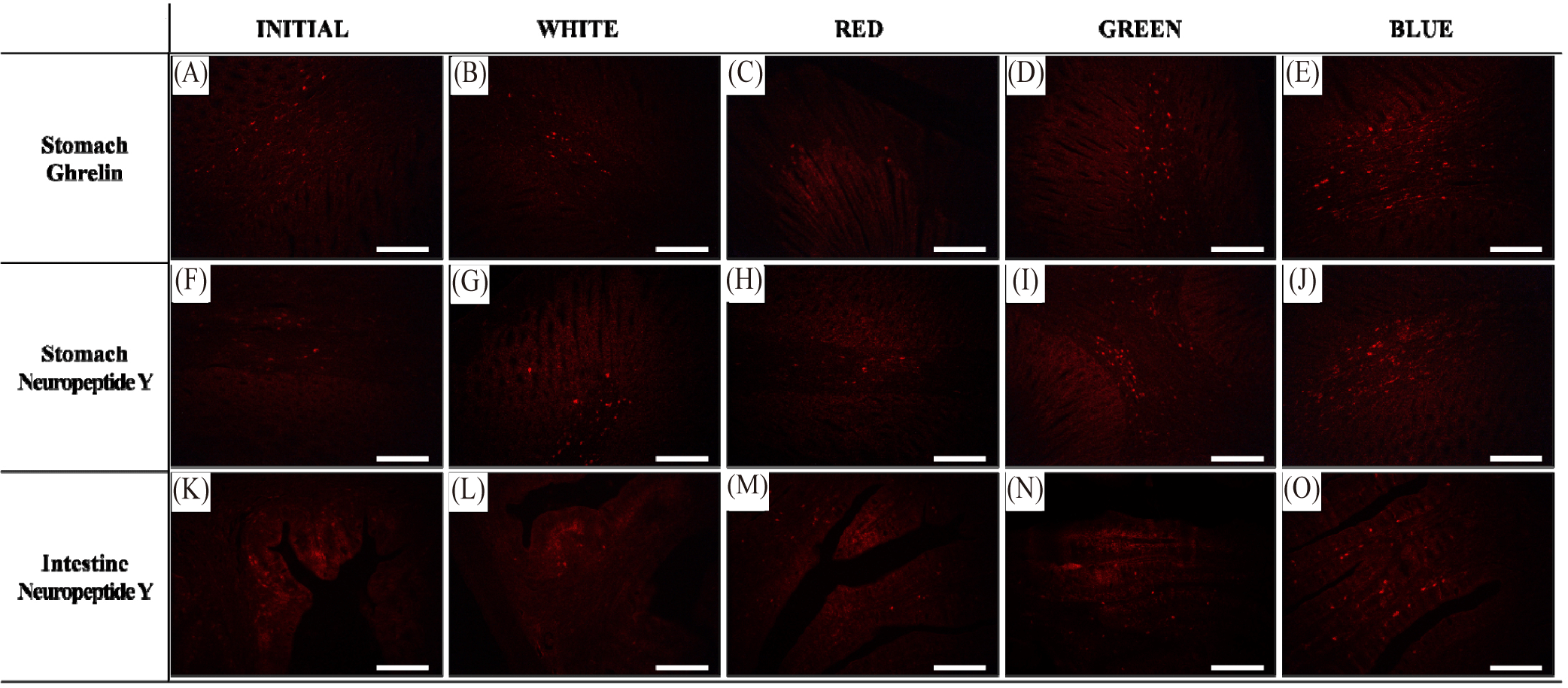
The expression of GHRL in the stomach and NPY in the stomach and intestine was observed following FISH analysis, and the results are shown in Fig. 2. Expression of GHRL was confirmed in the stomach of red seabream; wherein, fluorescence was most abundantly observed in the blue light treatment among other treatments, indicating high GHRL expression. In contrast, red light treatment induced a sparse fluorescence, demonstrating low GHRL expression. On the other hand, NPY expression was similarly detected in the stomach and intestine, and fluorescence was influenced by the different light treatments (Fig. 2). Most abundant fluorescence in both tissues was observed in the blue light, followed by green, white and red light treatments. Similar with GHRL expression, NPY expression was the highest in blue and lowest in red light.
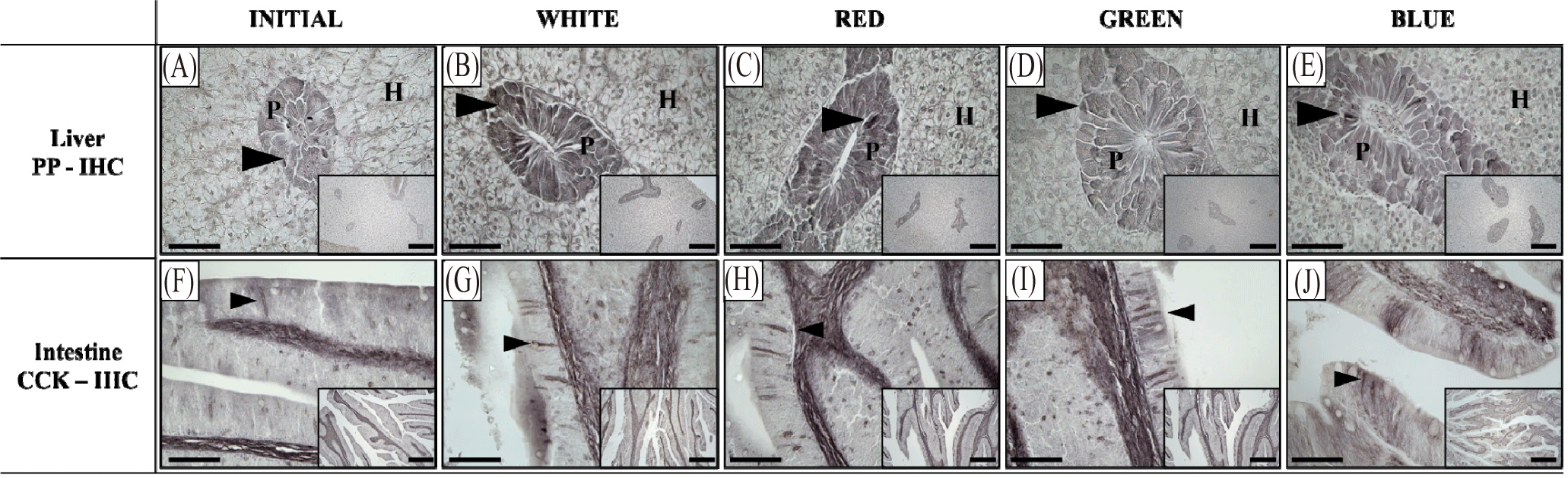
Detection of PP in the liver and CCK in the intestine was done following IHC method, and the results are shown in Fig. 3. Positive-stained PP cells were brightly-stained in the red and white experimental groups compared at the start of the study, in contrast to blue and green experimental groups wherein lighter staining was observed. On the other hand, positive signal of CCK was observed in the intestine of red seabream either as a brown-colored spindle-shaped or spherical shaped cells along the columnar epithelium of the intestinal tissue. While signal intensity was higher at the end of the culture period compared to the initial, no visually apparent difference on the structure and signal intensity were observed in the CCK-stained cells in all light treatments.
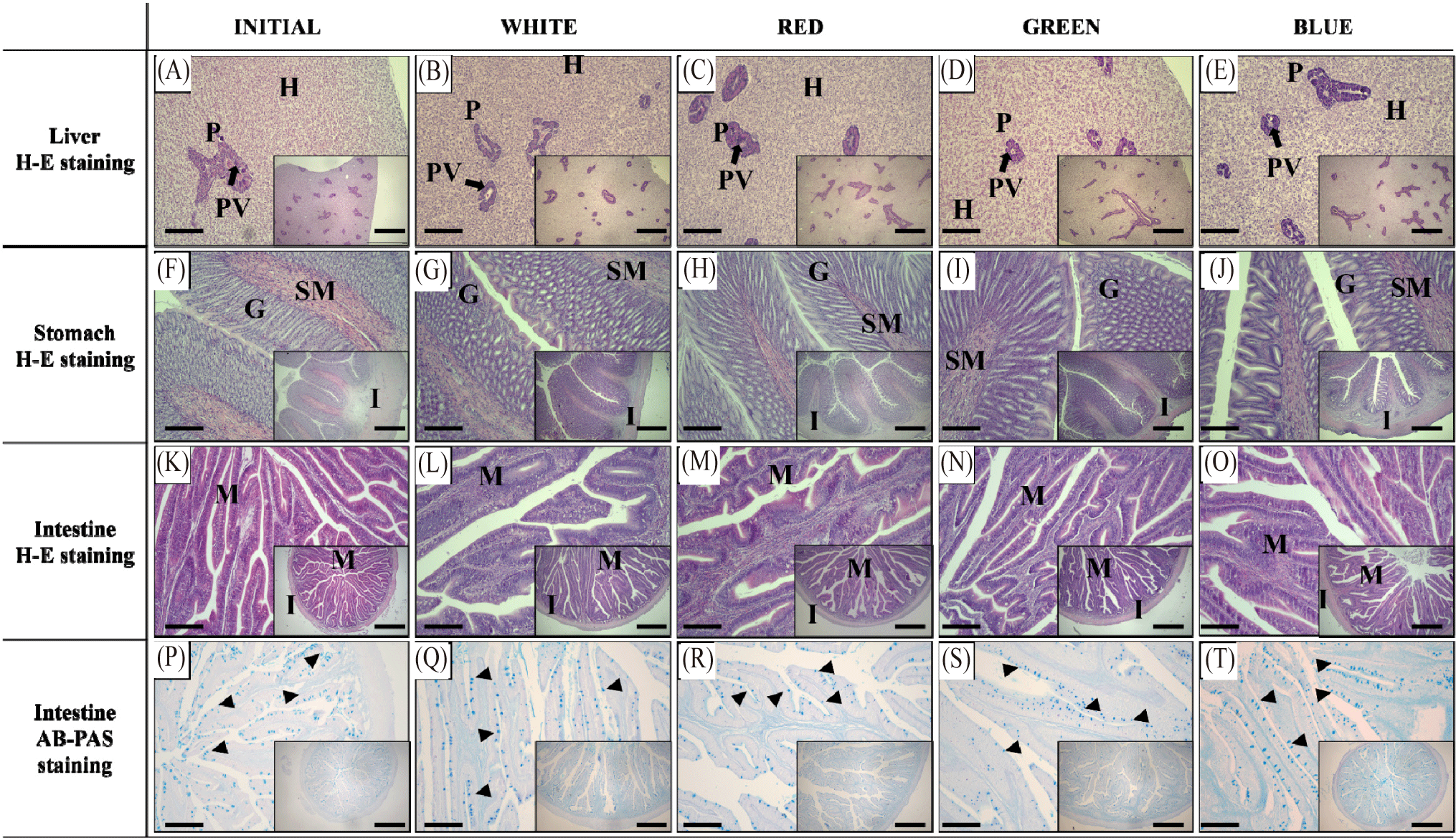
The histological analysis results of the liver, stomach, and intestine of red seabream following exposure to different LED spectra are presented in Fig. 4. In the liver tissues. The arrangement of hepatocytes and the development of the pancreas were denser compared to the start of the experiment (initial), but similar morphological structure was observed in all experimental groups. The distribution of pancreatic enzyme granules was also observed to be similar among the experimental groups (Fig. 4A–4E). In the stomach tissues, development of the muscle layer and submucosa was observed compared to the initial group; however, there were no describable morphological differences in the muscle layer, submucosa, and gastric glands among the experimental groups (Fig. 4F–4J). On the other hand, morphological arrangement of the intestinal villi was observed to be similar among experimental groups including at the start of the study (Fig. 4K–4O). Likewise, villi length and thickness of the muscle layer was not significantly affected by different light spectral experimental groups (p > 0.05, Table 2). The number of goblet cells per unit area was significantly lower in the Red experimental group (p < 0.05), while no significant differences were found in the Blue, Green, and White experimental groups (p > 0.05, Table 2).
Discussion
The distribution of neuropeptides aligns with the digestive physiology and feeding habits of fish species (Barrios et al., 2020). Regulation of appetite and digestive process are influenced by environmental factors such as exposure to different light wavelengths through positive or negative stimulation of various neuro- and gastro-endocrinological hormones (Motoike et al., 2016) and can affect growth performance of fish species. GHRL detects fasting conditions in the digestive system and sends signals to the hypothalamus to stimulate appetite and induce food intake. GHRL is primarily secreted from the stomach, but in some fish species that lack a stomach, secretion is from the intestine. Additionally, other tissues such as the pituitary gland, hypothalamus, pancreas, heart, thyroid, kidneys, liver, and immune system also produce GHRL, albeit in small amounts (Hosoda et al., 2000). The gut-brain axis is a bidirectional signaling system between the digestive tract and the central nervous system, particularly the hypothalamus. It regulates appetite, energy metabolism, gastrointestinal motility, and immune responses through hormones, neurotransmitters, and immune signals. Key components of the gut-brain axis include gastrointestinal endocrine cells, the vagus nerve, and gut microbiota, which detect signals from food and relay them to the central nervous system. Among neurogastroendocrine hormones, GHRL and NPY play significant roles in this signaling process, mediating appetite stimulation and energy metabolism regulation. GHRL is predominantly secreted during fasting, stimulating hypothalamic neurons to promote NPY secretion, which subsequently induces food intake (Blanco et al., 2021; Li et al., 2023). The cells that produce GHRL in the stomach account for 20% of the endocrine cell population, and the distribution decreases from the stomach towards the intestine (Date et al., 2000). On the other hand, NPY is secreted from the arcuate nucleus in the hypothalamus and acts as a potent appetite stimulant, interacting with other hormones in the hypothalamus and being expressed through the gut-brain axis (Holzer et al., 2012). In the peripheral nervous system of the digestive tract, NPY regulates autonomic nervous system responses, such as vasoconstriction, and plays roles in the regulation of gastrointestinal motility and secretion (Rindi et al., 2004; Unniappan & Peter, 2005). The FISH results of this study indicated that both GHRL secreted from the stomach and NPY secreted from the stomach and intestine exhibited differential expression in the order of blue, green, white, and red experimental groups. This suggests that blue light contributes to the activation of the HPA axis, inducing differential gene expression as growth progresses (Dempsey et al., 1943). Moreover, since green light has a weaker effect on regulating the biological clock compared to blue light, the increase in the expression of GHRL and NPY was confirmed to be less than that of blue light. White light contains various wavelengths and may show a mixed effect of blue and green light; however, it does not provide strong stimulation due to specific wavelengths, leading to a moderate increase in the expression of GHRL and NPY. Red light, being a longer wavelength that contrasts with blue light, has minimal effects on the biological clock of red seabream, thereby minimizing its impact on the expression of GHRL and NPY, which explains low expression. Furthermore, results of the current study concur with the growth of gilthead seabream (Sparus aurata) under different LED spectra (Karakatsouli et al., 2008). Thus, the continuous exposure of red seabream to blue light induces appetite the most in contrast to other wavelength exposures tested by regulating GHRL and NPY hormone expression. The increase in appetite (implying increase feed consumption) correlates to the increase in the growth performance of red seabream in blue light and the contrary in the red experimental group.
PP is exclusively expressed in the endocrine cells of the digestive system and regulates exocrine pancreatic function, inhibiting digestive enzyme secretion and intestinal motility, which induces satiety through its action on the hypothalamus in the central nervous system (Browning & Travagli, 2014; Katsuura et al., 2002; Williams, 2014). PP secretion is stimulated by food intake and amount is dependent on the digestive state of the animal (Jesudason et al., 2007). In the present study, IHC results showed that PP staining was darker in the red and white experimental groups compared to the green and blue experimental groups. While food intake stimulates PP secretion, overexpression of PP can also result to food intake suppression due to the delay of gastric emptying (Asakawa et al., 2003). This suggest that white and red LED light might have suppressed gastric emptying time causing continuous expression of PP in the pancreas of red seabream. However, we deemed that the exact role of LED and mechanism needs further investigations. Meanwhile, CCK is released into the bloodstream from the intestine and acts on the hypothalamus in the brain to induce appetite suppression and satiety; however, the expression of CCK did not show significant differences among LED spectrum exposure. This suggests that the effect of various LED exposure in the satiation of the red seabream was through PP regulation and not through CCK, and among light wavelengths, blue and green light induced reduction in PP secretion in the liver of red seabream.
In contrast to the observed differences in the body growth and expression of gastrointestinal hormones, the morphological structure of the liver, stomach and intestine was not affected following exposure to various LED wavelength in red seabream. An exception is the significantly lower distribution of goblet cells in the intestine observed in the red experimental group. Goblet cells are distributed in the mucosal epithelium, secreting mucus to protect cells and increase intestinal motility and digestive activity, thus promoting digestion and absorption capabilities (Willora et al., 2022). The number of goblet cells serves as an indicator to assess the health status of the intestine and associated histological changes (Leduc et al., 2018). The reduction in these goblet cells in the red experimental group suggests less efficient nutrient absorption and is believed to have impacted growth performance along with the decrease in appetite-related genes in the red experimental group.
LED exposure of red seabream impacted the fish appetite through regulation of various hormones without major morphological influence in the anatomy of different digestive organs. Specifically, blue light induced appetite through increased expression of GHRL and NPY and reduced PP-secretion in the liver which concur with its fast growth of the fish. This scenario was observed less or in contrary in the other LED experimental groups. Overall, the current study emphasized the possible usage of blue LED as lighting strategy to increase the appetite and growth of red seabream with potential application in an aquaculture setting.
