Introduction
In most cultures, alcohol consumption is customary; however, alcohol abuse has become a significant global issue. Statistics indicate that over 2 billion people consume alcohol regularly, with 3 million annual deaths attributed to excessive alcohol use – accounting for 6% of all fatalities worldwide (Bajaj, 2019). Alcohol intake is closely linked to alcohol-induced liver disease (ALD), a chronic condition associated with high mortality and morbidity rates. The detrimental effects of alcohol overconsumption are well-documented with ALD encompassing alcoholic fibrosis, fatty liver, steatohepatitis, and hepatitis (Cichoż-Lach & Michalak, 2014).
ALD is a complicated pathological process leading to hepatocyte death and inflammation, potentially resulting in liver failure (Zakhari, 2006). Alcohol-induced liver inflammation, or alcoholic hepatitis, represents the most severe form of alcoholic liver damage. Alcohol consumption markedly increases acetaldehyde production in the liver, which stimulates Kupffer cells and induces inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α), contributing to liver damage through various mechanisms (Dong et al., 2020). Toll-like receptors (TLRs), particularly TLR4, are activated by ethanol (Tilg et al., 2011), triggering nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPK) pathways. This activation promotes the production of inflammatory mediators, including TNF-α and IL-6 (Tilg et al., 2011). The upregulation of these mediators exacerbates hepatocyte damage and recruits inflammatory cells, leading to further tissue injury (Kono et al., 2001).
Current treatments for alcohol-induced liver injury include glucocorticoids, superoxide dismutase, and silymarin (Song et al., 2006; Wheeler et al., 2001). However, these treatments are often ineffective for acute liver damage and may have side effects. Recently, there has been growing interest in natural compounds as safer alternatives with fewer side effects. Natural substances that modulate the production of inflammatory factors could serve as effective anti-inflammatory agents. Styela clava, an ascidian tunicate, is cultivated in South Korea for its unique flavor and is also found globally. Previous studies have documented its antihypertension, anticancer, and antioxidant properties (Jumeri & Kim, 2011; Kim et al., 2006; Ko et al., 2012), along with its antioxidant potential derived from carotenoids (Nacional et al., 2011). Moreover, S. clava has demonstrated skin aging preventive activity by inhibiting related enzymes (Lee et al., 2015). However, its hepatoprotective effects against alcohol-induced liver inflammation remain unexamined. Thus, this study aims to explore the role of S. clava and its underlying molecular mechanisms in mitigating alcohol-induced liver inflammation. Our findings may support the potential use of S. clava in various applications, including the development of nutraceuticals and functional foods, offering significant insights into its utilization.
Materials and Methods
HepG2 cell line was obtained from the American Type Culture Collection (Rockville, VA, USA). Fetal bovine serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM), and penicillin-streptomycin were obtained from Gibco/BRL (Burlington, ON, Canada). Enzyme-linked immunosorbent assay kits for IL-1β, IL-6, and TNF-α were acquired from R&D System (Minneapolis, MN, USA). Methanol, chloroform, and BCA protein assay kit were purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary and secondary antibodies were from Santa Cruz Biotechnology, CA, USA. Enhanced chemiluminescence reagent was obtained from Amersham (Arlington Heights. IL, USA). Chemicals such as Hoechst 33342, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dichlorofluorescin diacetate (DCF-DA), acridine orange, and diaminofluorophore, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH2-DA), and 4-amino-5-methylamino-2,7-difluorofluorescein diacetate were purchased from Sigma-Aldrich. All other chemicals and solvents were of analytical grade obtained from Sigma-Aldrich.
S. clava was collected from Jeju Island in June 2021. The sample was washed to remove sand and other impurities. The flesh of S. clave was ground and extracted with 50% methanol. Briefly, 200 mL of 50% methanol was added to ground meat (20 g) and sonicated for 10 min. This process was repeated three times. The extract was then pooled and centrifuged at 1,120 ×g for 10 min. The supernatant was collected, concentrated using a rotary evaporator, and dried under a nitrogen stream. The dried extract (SCM) was then used for subsequent experiments.
HepG2 liver cells were cultured and maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin in a CO2 incubator. Cells exhibiting exponential growth were used for the experiments.
The cytotoxicity of SCM, ethanol, and their combination on HepG2 liver cells was evaluated using a colorimetric MTT assay, as described previously (Nagahawatta et al., 2023). HepG2 liver cells were seeded at a concentration of 1×105 cells/mL in a 96-well plate and incubated for 24 h. The cells were then treated with different concentrations of SCM (50, 100, 200, 400, and 800 µg/mL) and incubated for another 24 h in a CO2 incubator. Following this, 50 µL of MTT reagent was added to each well and incubated in the dark for 2 h. After incubation, the cell culture medium was replaced with 200 µL of dimethyl sulfoxide (DMSO) per well, and the optical density was measured using a microplate reader.
To determine the optimal ethanol concentration for stimulating liver injury, HepG2 liver cells were seeded as previously and treated with various concentrations of ethanol (1, 2, 3, 4, 5, and 6%). After a 24 h incubation, the MTT assay was conducted (Dai et al., 2020). For analyzing the protective effect of SCM on alcohol-induced liver cell viability, HepG2 liver cells were seeded as previously mentioned and treated with SCM. After 1 h of incubation, the cells were stimulated with 3% ethanol and further incubated for 24 h, after which cell viability was measured using MTT assay (Liyanage et al., 2022c).
The capacity of SCM to scavenge alcohol-induced intracellular reactive oxygen species (ROS) was determined using the DCF-DA assay (Liyanage et al., 2022c). HepG2 liver cells were seeded in a 96-well plate and incubated for 24 h. The cells were then pretreated with SCM (50, 100, 200, 400, and 800 µg/mL) for 2 h prior to the stimulation with 3% ethanol. After 24 h, the cells were treated with DCF-DA (5 µg/mL) and further incubated for 10 min in the dark. The fluorescent intensity of the media was measured using a microplate reader (Bio-Tek, Winooski, VT, USA) at excitation and emission wavelengths of 485 and 535 nm, respectively.
The nucleus morphology of cells was examined using the DNA dye Hoechst 33342 to identify apoptosis caused by 3% ethanol treatment (Lee et al., 2023). HepG2 liver cells were seeded at a concentration of 1×105 cells/mL and incubated for 24 h in a CO2 incubator. The cells were then pretreated with SCM (100 and 200 µg/mL) for 2 h, followed by stimulation with 3% ethanol. After an additional 24 h of incubation, Hoechst 33342 was applied to the cells at a final concentration of 10 µg/mL, and they were incubated for another 10 min. A fluorescence microscope (Olympus, Tokyo, Japan) was used to detect stained cells, and ImageJ software was employed to calculate the apoptosis levels in each sample.
HepG2 liver cells were seeded and incubated with SCM and 3% ethanol as described in section 2.3.3. The cell culture supernatants were collected, and the expression levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) were measured using ELISA kits following the manufacturer’s instructions (Nagahawatta et al., 2022).
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in the liver were assessed using commercial assay kits according to the manufacturer’s instructions. In brief, cells were incubated in the presence or absence of SCM for 2 h and then stimulated with 3% ethanol for another 24 h. After that, the cell lysate was prepared, and the ALT and AST levels were measured using a microplate reader at 340 nm (Kumar et al., 2011).
The effect of SCM on alcohol-induced liver inflammation was analyzed by measuring the protein expression levels of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), NF-κB, the MAPK pathway, TLR4, and NLRP3 using Western blot analysis (Liyanage et al., 2022b). Briefly, HepG2 liver cells were preincubated with SCM and stimulated with 3% ethanol. Cells were harvested, and their proteins were extracted. Protein concentrations were determined using a BCA protein assay, separated by 12% SDS-polyacrylamide gel electrophoresis, and transferred onto a nitrocellulose membrane. The membranes were pre-blocked with 5% skim milk before being incubated with primary and secondary antibodies. Finally, a chemiluminescent substrate (Cyanagen Srl, Bologna, Italy) was used to visualize the proteins using FUSION SOLO Vilber Lourmat system (Paris, France). The density of each band was quantified using ImageJ software.
Adult zebrafish were obtained from a commercial supplier (Seoul Aquarium, Seoul, Korea) and interbred. The fish were fed with a commercial diet twice a day and allowed to mate. Fertilized embryos were collected and used in subsequent experiments. This experiment was conducted in accordance with the experimental animal guidelines provided by the Jeju National University Animal Center and approved by the Animal Care and Use Committee of Jeju National University (protocol 2020-0049). All experiments using zebrafish larvae were conducted in triplicates.
Zebrafish embryos at 7–9 h post-fertilization were placed in a 12-well plate (15 embryos per well) and exposed to SCM (100 and 200 µg/mL) for 1 h. Subsequently, a fresh medium containing 3% ethanol was then added. The survival rate of the treated embryos was monitored over 7 days. Hatched embryos were maintained under continuous observation, and the heart rates of the hatched larvae were measured at 2 days post-fertilization (dpf) (Dai et al., 2020).
The 3 dpf larvae were used in the in vivo experiments as previously described (Liyanage et al., 2022b). Alcohol-induced NO production was measured using DA-FMDA, and ROS production was measured using DCFH2-DA (20 µg/mL). For the measurement of cell death, acridine orange staining (7 µg/mL) was used. Briefly, 3 dpf zebrafish larvae were treated with SCM (100 and 200 µg/mL) for 2 h and then induced with 3% ethanol for 24 h. Following staining, the zebrafish larvae were rinsed with embryo medium and anesthetized by immersion in E3 medium containing 70 µg/mL tricaine (ethyl 3-aminobenzoate, Sigma-Aldrich). The fluorescence microscope was used to visualize the stained larvae, and the obtained data were analyzed using ImageJ software. The experiments were done in triplicate.
The compounds in SCM were identified by gas chromatography/mass spectrometry (GC/MS) analysis using an Agilent Head Space-Gas Chromatography-Tandem Mass Spectrometer system (Agilent Technologies, CA, USA) (Liyanage et al., 2022a). An SLB-5ms Capillary GC Column (30 m×0.25 mm, 0.25 µm, Supelco Inc, Sigma-Aldrich) was used in the GC-MS. In a splitless mode. Helium was employed as the carrier gas in splitless mode at a flow rate of 0.6 mL/min. After stabilizing for 5 min, the oven’s temperature was raised to 220°C at a rate of 2°C/min. The temperature was raised to 300°C and maintained there for 1.8 min after stabilizing for another 5 min. Compounds were identified by comparing their mass spectra with data from NIST 11.
Statistical analysis was performed using GraphPad Prism 9 software (GraphPad Software, San Diego, CA, USA), and the results were expressed as the mean ± standard deviation (SD) of triplicates. Mean values were compared using one-way analysis of variance (ANOVA). Duncan’s multiple-range test for was used for mean separation.
Results
The toxicity of SCM on HepG2 liver cells was measured using MTT assay. As shown in Fig. 1A, there was no marked decrease in cell viability when treated with various concentrations of SCM (50, 100, 200, 400, and 800 µg/mL). To determine the cytotoxic effect of ethanol on HepG2 liver cells, different concentrations of ethanol were applied to the cells. As shown in Fig. 1B, ethanol treatment decreased cell viability. Notably, treatment with 3% ethanol reduced cell survival to 53.78%. Therefore, a 3% ethanol concentration was selected for further studies.
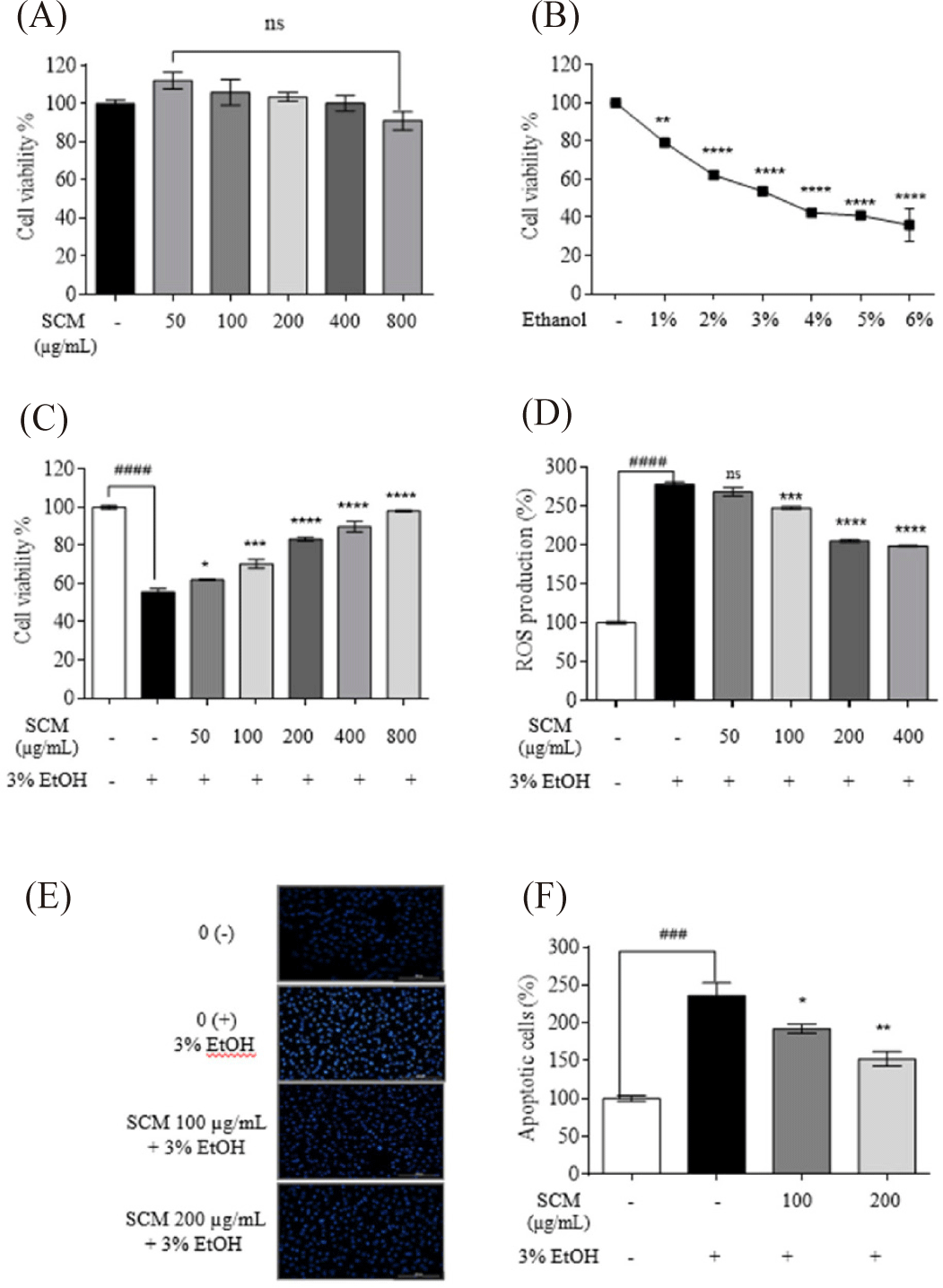
To assess the protective effect of SCM on cell viability, HepG2 cells were stimulated with 3% ethanol. The results indicated that the drastically decreased cell viability (55.60 ± 1.81%) due to ethanol stimulation was recovered with SCM treatment in a dose-dependent manner (Fig. 1C). Similarly, 3% ethanol treatment stimulated intracellular ROS production in cells (277.25 ± 3.13%). The increased ROS production was significantly reduced with SCM treatment in a dose-dependent manner (Fig. 1D). Based on the obtained results, 50 µg/mL did not exhibit any significant ROS inhibition. Therefore, the lowest tested SCM concentrations of 100 and 200 µg/mL were selected for further studies.
The nuclear morphology of the cells was examined using Hoechst 33342 staining (Fig. 1E and 1F). Increased chromatin condensation and DNA fragmentation in 3% ethanol-treated cells were indicated by higher fluorescence intensity in the nuclei. With dose-dependent treatment of SCM, the fluorescence intensity decreased, indicating a reduced number of apoptotic bodies. This was further confirmed by measuring the percentage of apoptotic cells using ImageJ software. The 3% ethanol treatment resulted in 236.35 ± 16.58% of apoptotic body formation whereas 100 and 200 µg/mL concentrations of SCM treatment resulted in decrease in the apoptotic bodies up to 192.15 ± 6.00% and 152.45 ± 9.43%, respectively.
IL-6, IL-1β, and TNF-α expression have been widely studied for their involvement in both acute and chronic liver inflammation (Shin et al., 2013). As shown in Fig. 2A–2C alcohol treatment increased the production of IL-6, TNF-α, and IL-1β in HepG2 liver cells compared to the untreated control group. However, treatment with SCM significantly decreased their upregulated production.
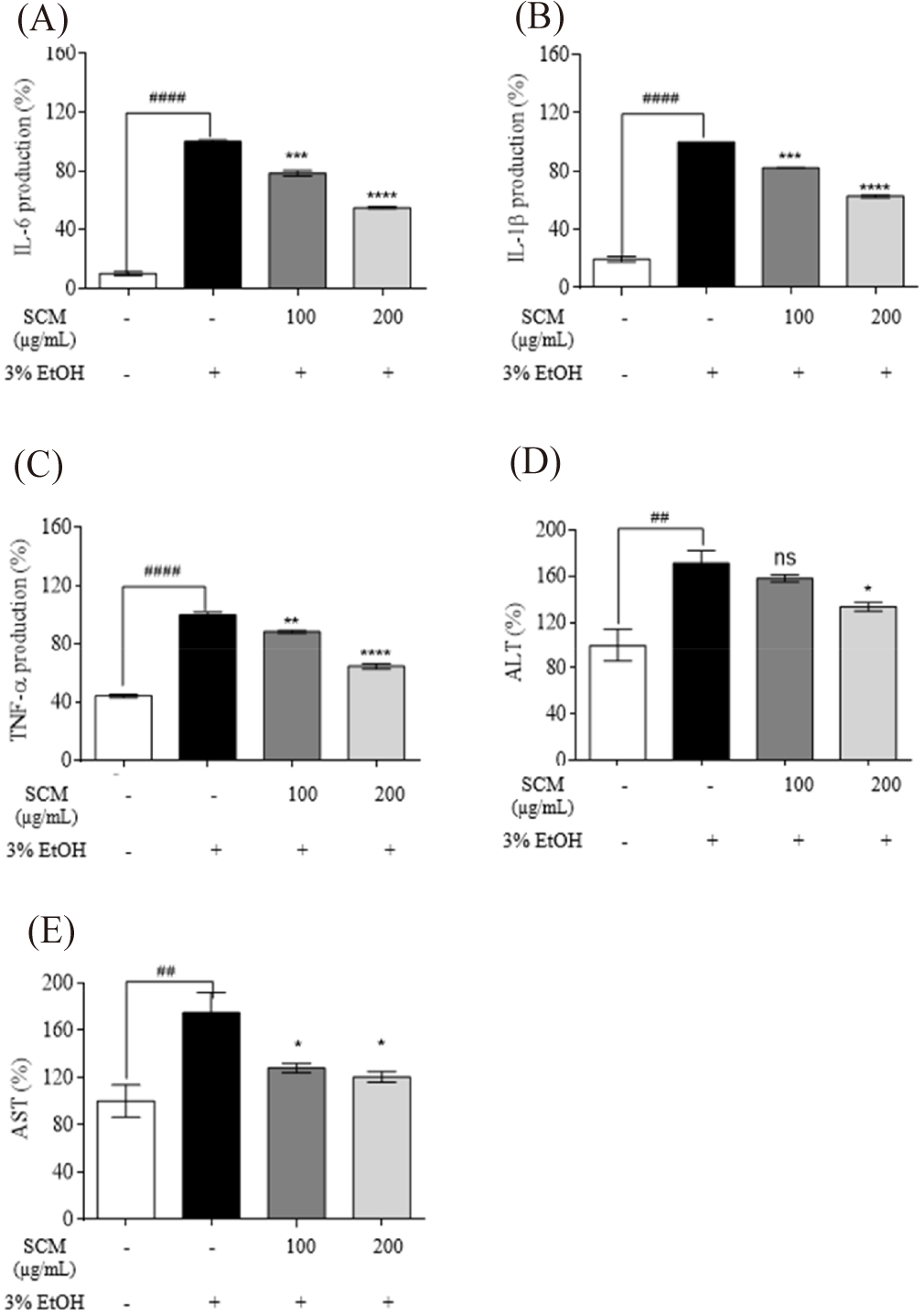
The ALT and AST levels in 3% ethanol-induced HepG2 liver cells were measured to assess the protective impact of SCM against liver damage. As shown in the Fig. 2D and 2E, ethanol significantly increased ALT and AST levels by 171.20 ± 11.10% and 174.20 ± 11.10%, respectively, compared to the untreated group. However, these increased ALT was reduced to 158.25 ± 3.16% and 133.51 ± 3.70% with the treatment of 100 and 200 µg/mL concentrations of SCM, whereas AST production was decreased to 128.06 ± 3.91% and 120.37 ± 4.67%, respectively.
Western immunoblot analysis was used to examine the expression levels of iNOS and COX-2 proteins in alcohol-induced HepG2 liver cells (Fig. 3A and 3B). The results demonstrated that 3% ethanol stimulation increased iNOS and COX-2 levels compared to the untreated group. The treatment with SCM lowered the upregulated of iNOS and COX-2 in HepG2 liver cells in a concentration-dependent manner, supporting its anti-inflammatory activity. The iNOS expression was downregulated by 0.83 folds and 0.48 folds, while COX-2 expression was decreased by 0.96 folds and 0.47 folds by 100 and 200 µg/mL of SCM, respectively.
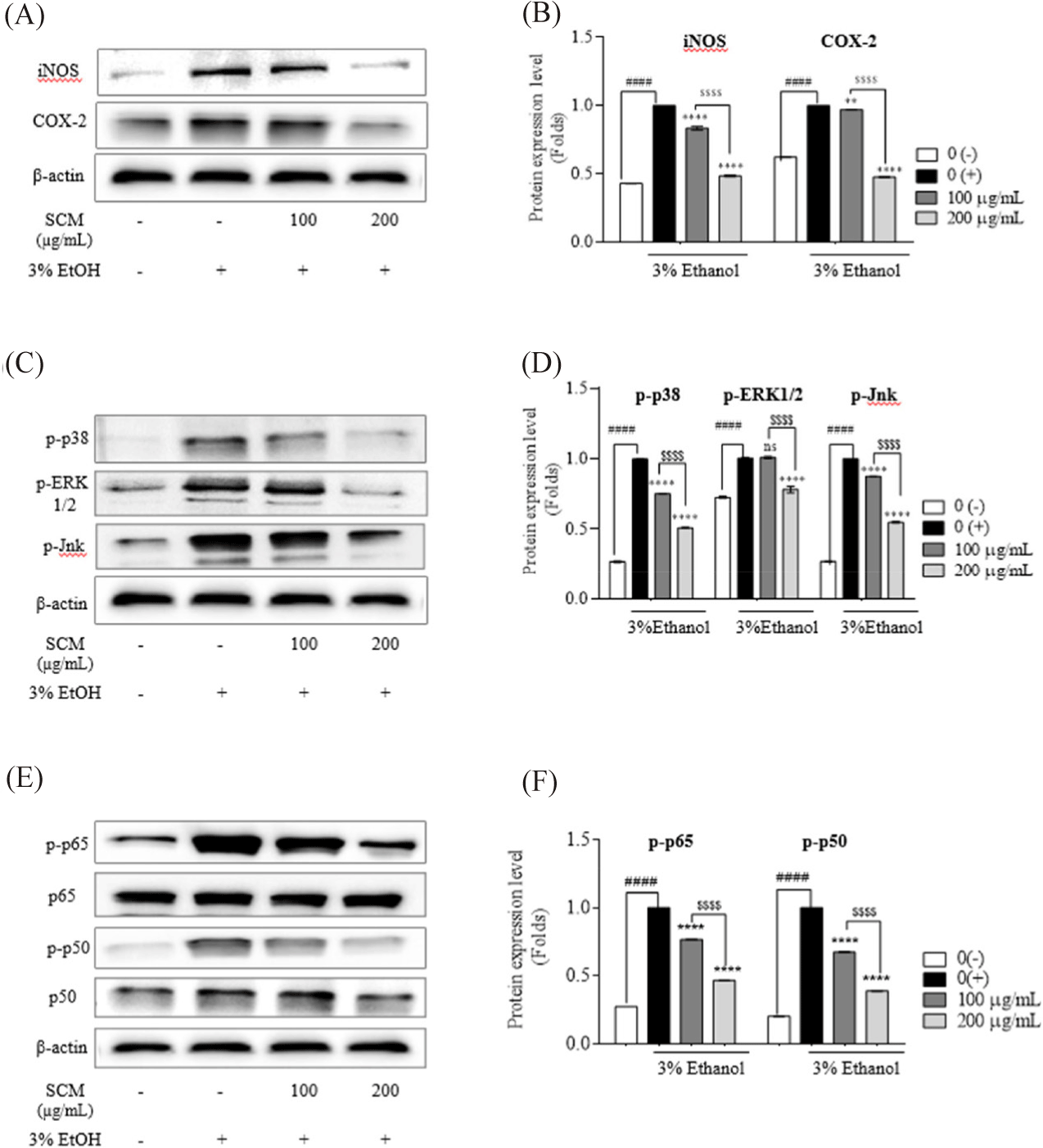
To further study the underlying mechanism by which SCM inhibits liver damage induced by ethanol treatment, the expressions of key inflammation-related proteins in the NF-κB and MAPK pathways were analyzed by Western blot. Gene expression and cytokine production are induced by the phosphorylation of transcription factors such as c-Jun N-terminal kinase (Jnk), ERK1/2, and p38. As shown in Fig. 3C and 3D, 3% ethanol stimulation increased the phosphorylation of MAPK family proteins in HepG2 liver cells compared to untreated cells. Interestingly, the upregulated protein expressions of phosphorylated (p)-p38, p-Jnk and p-ERK1/2 were suppressed by SCM treatment in a dose-dependent manner. Treatment with 100 µg/mL and 200 µg/mL of SCM resulted in a 0.75- and 0.5-fold reduction in p-p38, and a 0.87- and 0.54-fold reduction in p-JNK protein expression, respectively. However, 100 µg/mL SCM did not reduce the p-ERK 1/2 expression.
As shown in Fig. 3E and 3F, the activation of NF-κB pathway was stimulated by 3% ethanol treatment in HepG2 liver cells, resulting in the increased phosphorylation of the p50/p65 subunits. However, pre-treatment of the cells with SCM downregulated their phosphorylation in a concentration-dependent manner. The treatment of 100 and 200 µg/mL SCM resulted in 0.76- and 0.46- fold reduction in p-p65, and a 0.67- and 0.39-fold reduction in p-p50 protein expression, respectively. These findings demonstrate that SCM significantly reduces NF-κB and MAPK phosphorylation in ethanol-stimulated HepG2 liver cells, indicating strong anti-inflammatory activity.
The protective effects of SCM on 3% ethanol-treated zebrafish were evaluated. First, SCM samples up to 400 µg/mL concentration showed no significant toxic effect on zebrafish embryos (Fig. 4A). Therefore, at dosages of 100 and 200 µg/mL, the protective efficacy of SCM against 3% ethanol-induced toxicity in zebrafish was assessed. Treatment with 3% ethanol resulted in a drastic decrease in the survival rate of the embryos (33.33%) (Fig. 4B). However, pre-incubation with 100 and 200 µg/mL of SCM showed a remarkable protective effect, increasing embryo survival to 75% and 60%, respectively. Furthermore, stimulation with 3% ethanol increased the heartbeat rate of zebrafish larvae compared to untreated larvae. The increased heartbeat rate was recovered with the dose-dependent treatment of SCM (Fig. 4C).
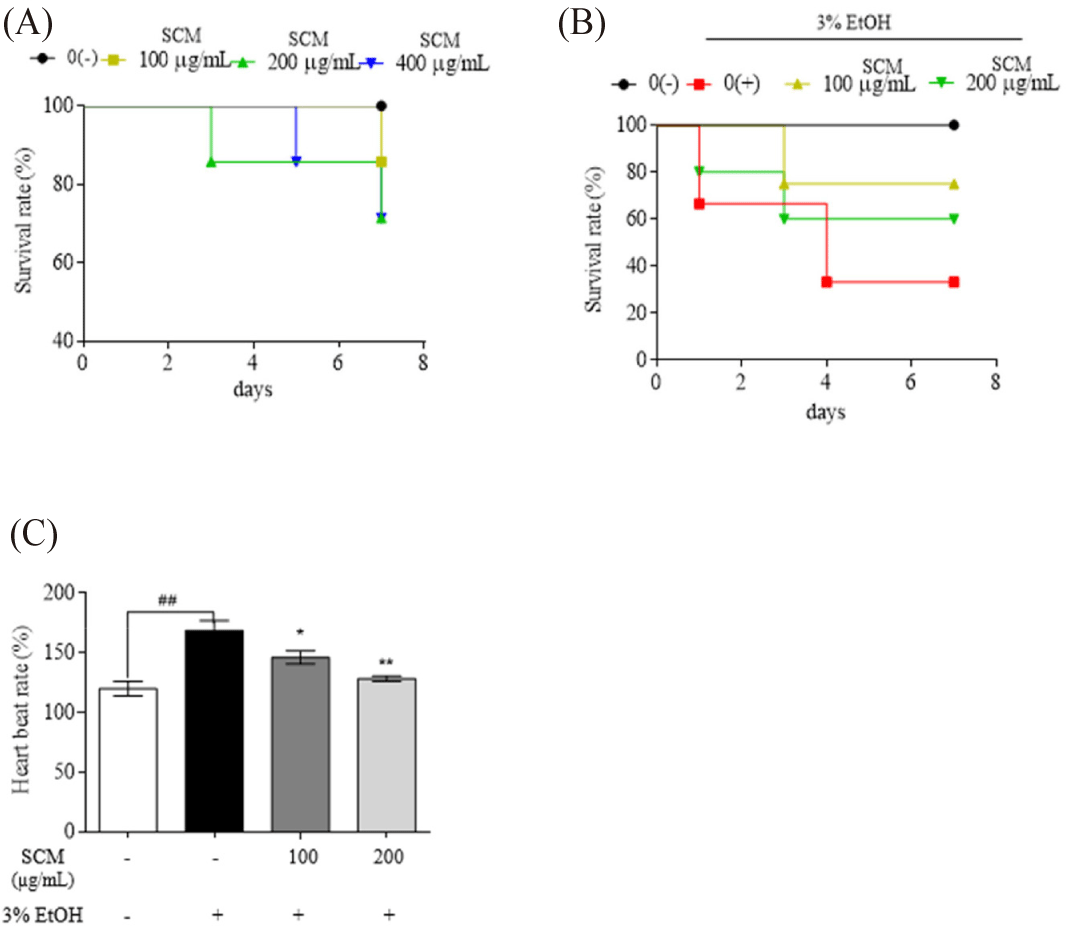
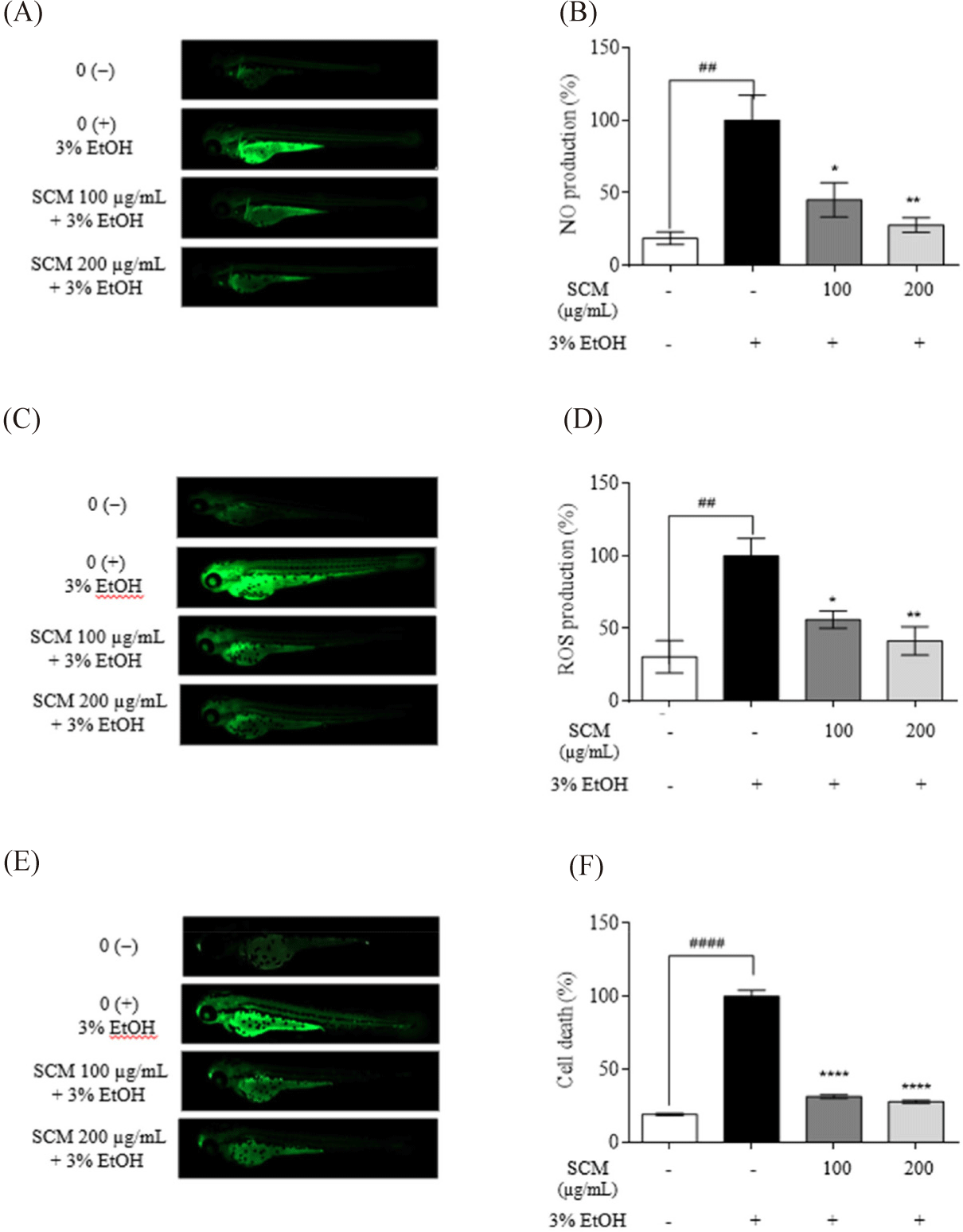
As shown in Fig. 5A, NO production in 3% ethanol-induced zebrafish larvae was increased compared to that in the untreated group. With SCM treatment at 100 and 200 µg/mL, NO production decreased significantly (45.34 ± 11.85% and 37.84 ± 5.06%, respectively). Moreover, the stimulated larvae exhibited increased ROS generation, which was repressed by the treatment with SCM (Fig. 5B). Alcohol-induced cell death in zebrafish was determined using acridine orange staining (Fig. 5C). Based on the result, strong fluorescence was observed in the 3% ethanol-induced group, which declined significantly after dose-dependent treatment with SCM, reducing to 31.29 ± 1.15% and 27.91 ± 1.03%, respectively, with 100 and 200 µg/mL of SCM.
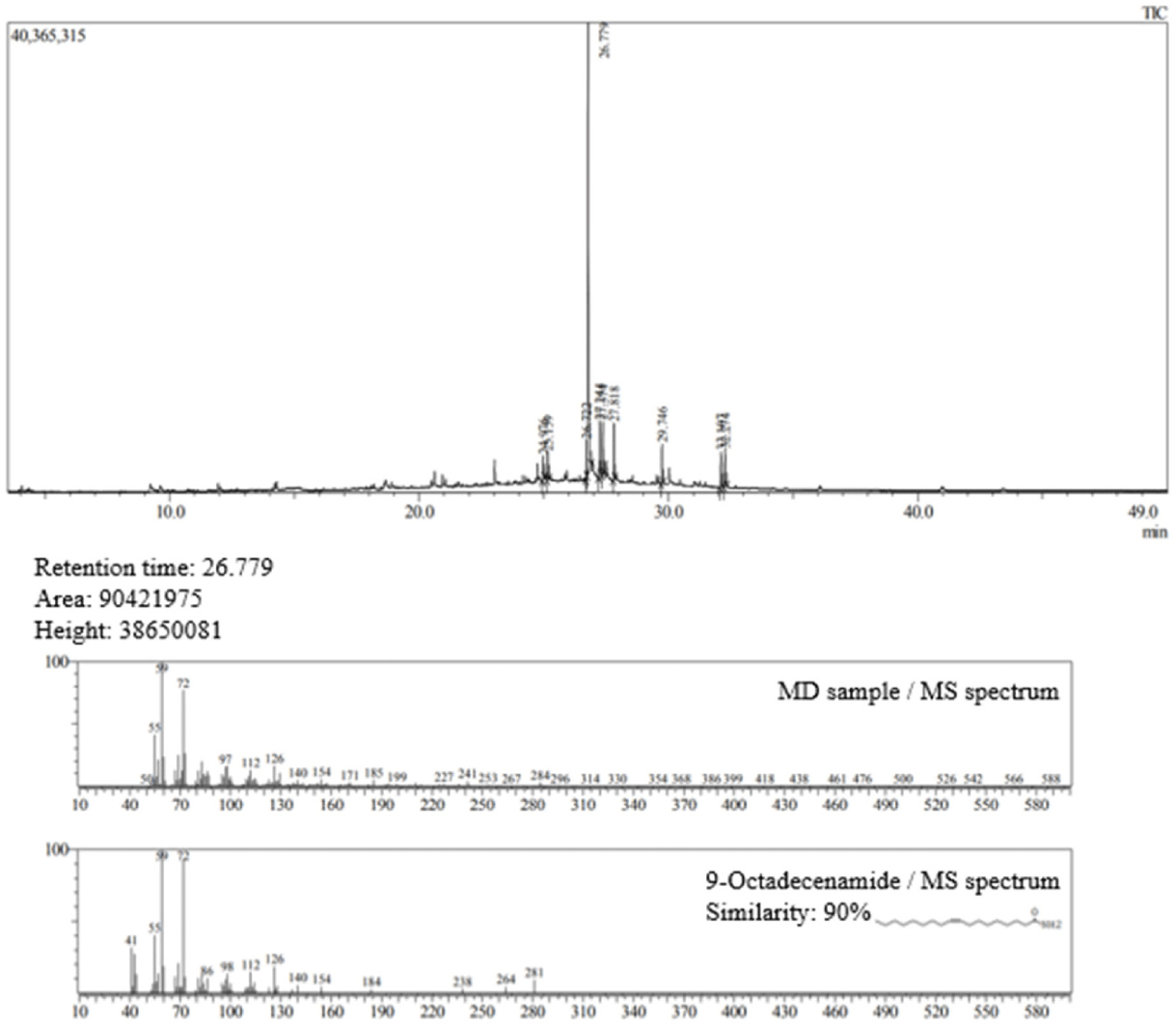
SCM was investigated using GC-MS. The highest peak was observed at the retention time of 26.779 min, and the compound was identified as 9-octadecenamide (Fig. 6), with a similarity of 90% to the database. This compound was identified as the most abundant in SCM.
Discussion
Much research has described the pathophysiology of ALD, highlighting the urgent need for additional medications with fewer side effects and toxicities for clinical therapy. Understanding the fundamental processes that cause liver damage is crucial for developing effective treatments to prevent or treat ALD. Evidence suggests that alcohol-induced liver damage is exacerbated by increased of NF-κB dependent gene expression in liver cells.
One commonly used therapeutic agent for ALD is silymarin, extracted from milk thistle. Reports indicate that silymarin reduces alcohol’s effect on the liver by blocking the NF-κB signaling pathway and lowering excess oxygen free radicals (Zhang et al., 2013). However, silymarin can cause gastrointestinal issues such as diarrhea, constipation, nausea, and headaches (Piscitelli et al., 2002). Therefore, there is a need for safe natural compounds to treat ALD.
In the present study, both in vitro and in vivo models were employed to investigate the direct effects and the mechanisms of S. clava methanol extract (SCM) in ethanol-induced liver damage. HepG2 liver cells were used as a non-malignant cell model for studying ethanol toxicity. The findings indicated that SCM had anti-inflammatory effects, reducing acute liver damage caused by ethanol. The underlying mechanism may be related to decreased production of IL-1β, TNF-ɑ, and IL-6 through the inhibition of the NF-κB and MAPK signaling pathways.
During alcohol consumption, the liver metabolizes alcohol. However, excessive alcohol intake can damage gut barrier function, leading to endotoxin production (Rao, 2008). In our study, ethanol treatment significantly decreased liver cell viability, indicating cell damage, while SCM treatment promoted improvement in cell viability. Apoptosis is a critical mechanism in the pathophysiology of liver disorders. The most significant apoptotic mechanisms implicated in ethanol-induced liver damage include death receptor activation, mitochondrial damage, and endoplasmic reticulum (ER) stress (Wang et al., 2006). Thus, preventing apoptosis in liver cells is advantageous in combating ALD. Our findings revealed that SCM effectively suppressed ethanol-induced apoptosis in liver cells. Similar results were found in a previous study showing the protective effect of L-theanine on alcoholic liver injury (Li et al., 2012). Another study reported the protective effect of fucoidan extracted from Sargassum fusiformis on ethanol-induced HepG2 liver cells (Dai et al., 2020).
ROS production occurs in the mitochondria and plays a major role in alcohol-induced cell injury. Increased ROS levels overwhelm cellular defensive mechanisms, resulting in oxidative stress and disease onset (Wu et al., 2006). In our study, cells exposed to ethanol showed a marked increase in ROS production, which SCM effectively reduced. These findings align with previously published reports (Dai et al., 2020; Wu et al., 2006). Endotoxins produced from alcohol consumption activate the production of pro-inflammatory cytokines in liver cells, contributing to liver injury. Previous studies have demonstrated that inflammation plays a major role in liver damage through the production of IL-1β, TNF-ɑ, and IL-6 (Wang et al., 2017; Xiong et al., 2017). Consistent with these reports, our study showed that ethanol pretreatment increased pro-inflammatory cytokine production, while SCM treatment effectively reduced pro-inflammatory cytokine levels in HepG2 liver cells (Yang et al., 2020).
Hepatocyte cytoplasm contains the soluble enzymes AST and ALT. Following hepatocyte damage, the cell membrane becomes more permeable, allowing ALT and AST to enter the bloodstream. Thus, serum ALT and AST levels serve as sensitive markers for liver damage severity. In our study, ethanol treatment increased ALT and AST levels in liver cells, while SCM reduced these levels. These findings suggest that SCM can modulate ALT and AST levels, thereby mitigating alcohol-induced liver injury. This hypothesis is supported by findings from earlier studies (Han et al., 2018; Zheng et al., 2019).
NO production plays a significant role in immunological modulation and is closely associated with iNOS and COX-2 levels (Liyanage et al., 2022b). Typically, higher iNOS levels coincide with COX-2 overexpression during inflammatory processes (Suliburk et al., 2005). COX-2 expression is stimulated by various inflammatory stimuli and is involved in inflammation, fibrous progression, and cancer development in the liver (Ma et al., 2011). Our study revealed that ethanol treatment markedly increased the iNOS and COX-2 expression, while SCM treatment lowered their expression levels. Similar results were observed in other studies examining the anti-inflammatory activity of quercetin and kaempferol against iNOS and COX-2 synthesis in HepG2 liver cells (García-Mediavilla et al., 2007; Suliburk et al., 2005).
Inflammation is a key factor in ALD development (Koyama & Brenner, 2017). The inflammatory process is regulated via several signaling pathways, including MAPK and NF-κB. MAPK, a serine- threonine protein kinase comprising ERK, p38, and JNK, plays a significant role in ethanol-induced liver cell apoptosis (Aksam et al., 2017). MAPKs are involved in developing oxidative stress and inflammatory responses. Our Western blot results revealed that ethanol stimulation activated MAPK pathway in HepG2 liver cells. SCM treatment downregulated the protein expressions of ERK, p38 and JNK in ethanol-induced liver cells, indicating SCM’s potential to inhibit the MAPK pathway. These findings align with previous studies demonstrating the downregulation of MAPK pathway through hepatoprotective effects of various compounds in alcohol-induced liver injury, both in vitro and in vivo (Li et al., 2017; You et al., 2020).
The regulatory mechanism of SCM on liver inflammation warrants further investigation, particularly concerning changes in the NF-κB signaling pathway. Alcohol overconsumption activates the NF-κB pathway, resulting in the secretion of inflammatory factors (Liu et al., 2017). The inactive form of NF-κB (p50/p65/IκB) resides in the cytoplasm. Upon stimulation, IκB phosphorylation occurs, leading to the translocation of phosphorylated p50 and p65 into the nucleus, where they bind to DNA and induce the synthesis of genes encoding inflammatory mediators (Liyanage et al., 2022b). Our study identified that ethanol stimulation resulted in the phosphorylation of p50 and p65. Similar findings were reported in studies examining the protective effects of quercetin against liver damage (Zhu et al., 2017). SCM prevented the activation of both MAPK and NF-κB pathway proteins in a concentration-dependent manner. These results are consistent with those of other studies on the protective effects of various natural compounds against alcohol-induced liver damage (Lin et al., 2021; Nie et al., 2021).
Zebrafish are widely served as valuable model organisms for investigating various diseases, owing to their functional and structural similarities to humans (Liyanage et al., 2022b). In this study, zebrafish were used to examine the anti-inflammatory potential of SCM in an in vivo model. Our findings demonstrated that acute ethanol exposure decreases zebrafish embryo survival while increasing ROS and NO production and cell death. Cell death and NO production play essential roles in maintaining homeostasis. The production of various cytokines, immune system activation, and inflammation can lead to tissue damage accompanied by cell death (Liyanage et al., 2022c). Our results confirmed the protective effect of SCM against ethanol-induced damage in zebrafish larvae, consistent with previous studies on the mechanisms involved in alleviating alcohol-induced liver damage (Galicia-Moreno & Gutiérrez-Reyes, 2014; Lai et al., 2018).
GC/MS indicated that SCM contained a higher amount of 9-octadecenamide, an oleamide that acts as a bioactive lipid signaling molecule in various organisms. Previous reports have demonstrated that this compound possesses anti-inflammatory activity (Hernández-Zazueta et al., 2021; Yue et al., 2019). One study showed that 9-octadecenamide decreased nitrite concentration and PGE2 production in LPS-induced BV2 murine microglial cells by inhibiting NF-κB pathway activation (Oh et al., 2010). Our results align with this earlier work.
Given the global prevalence of liver disease worldwide, including ALD, the development of safe and effective therapeutic strategies is crucial. Natural product-derived compounds, such as S. clava extracts, offer a promising alternative to conventional medicines, which often have adverse effects. Moreover, the use of natural products aligns with the growing interest in complementary and alternative medicine.
However, further preclinical and clinical studies are necessary to validate the efficacy and safety of S. clava methanolic extract in humans. Additionally, determining the optimal dosage, duration, and route of administration is crucial for translating these findings into clinical applications. Overall, our study contributes to the expanding body of research supporting the potential of natural products in the prevention and treatment of liver diseases, highlighting S. clava methanolic extract as a promising candidate for further exploration.
Conclusion
In conclusion, the present study provides compelling evidence supporting the potential of SCM as a therapeutic agent against ethanol-induced liver damage through the inhibition of inflammatory pathways. Mechanistically, SCM protects liver cells from alcoholic liver injury primarily by inhibiting apoptosis, reducing pro-inflammatory cytokine production, decreasing ROS levels, and inhibiting the MAPK and NF-κB signaling pathways. Furthermore, SCM demonstrated its protective effects in an ethanol-induced in vivo zebrafish model. These findings underscore the promising hepatoprotective properties of SCM and support its potential as a natural remedy for preventing and treating ethanol-induced liver damage. Identifying the specific bioactive compounds responsible for these effects warrants further investigation to elucidate the underlying mechanisms.
