Introduction
Freshwater crayfish (Cherax quadricarinatus) is a species originating from rivers and lakes in the tropical northern region of Australia (Rigg et al., 2020), and was introduced to Indonesia in 1991. According to Leeman et al. (2022), freshwater crayfish have several advantages, such as adaptability, ease of cultivation, and relatively high selling prices. However, there are challenges in the growth stage, as the development and physical form of male is faster compared to female due to several biological and behavioral factors (Adiputra et al., 2020). One primary reason is that male crayfish tend to be more aggressive and territorial, which often leads to better access to food resources (Su et al., 2024). This increased food intake supports faster growth. Additionally, males typically have larger claws and bodies, which are advantageous in mating competitions and territorial disputes (Heuring & Hughes, 2022). As reported by Wang et al. (2014), the relationship between length and weight showed significant differences between juvenile male and female crayfish when reared separately. These differences became particularly evident at 60 and 75 days, with body length considered as a covariate. This phenomenon is attributed to the faster development of chelae in males compared to females, which occurs as the crayfish reach a certain body length and begin to exhibit secondary sexual characteristics. Therefore, male individuals need to be prioritized for development to reduce the cultivation time.
In this context, a potential solution that has been proven effective in increasing the percentage of male freshwater crayfish is sex reversal method. According to Smith et al. (2023), sex reversal method changes the genotypic sex of female into a functional male or vice versa. Sex reversal techniques in aquaculture, while beneficial for increasing production efficiency, can have significant ecological implications. These techniques often involve manipulating the sex of fish to produce monosex populations, typically all-male or all-female, which can enhance growth rates and uniformity in size. However, the broader ecological impacts need careful consideration. One potential ecological implication is the risk of genetic diversity reduction. Monosex populations, if accidentally released into the wild, could disrupt natural breeding patterns and reduce genetic variability, which is crucial for the resilience of wild populations (Huang et al., 2023). Additionally, the use of hormones and other chemicals to induce sex reversal can have unintended effects on non-target species and ecosystems if not managed properly (Jamal et al., 2024).
This is carried out through hormone stimulation and chromosome manipulation, but the most used method in cultivation is the application of steroid hormones during the sex differentiation phase. Hormone application for sex reversal in fish can be performed through injection, immersion, and oral methods (da Silva Farias et al., 2023). According to the Minister of Marine Affairs and Fisheries Regulation No. 1 of 2019, the use of 17α-methyltestosterone (MT) has been restricted due to the potential environmental pollution and carcinogenic properties. Therefore, an alternative substance from natural sources is needed to replace the testosterone hormone such as the extract of rujak polo (Tribulus terrestris). Based on previous research, TT contains steroid saponin compounds, such as protodioscin, which play a role in biological activities as an aphrodisiac or sexual arousal (Singh et al., 2023).
This compound can increase the amount of luteinizing hormone (LH) produced by the pituitary gland to stimulate testosterone hormone secretion (Kho et al., 2023). Sarida et al. (2025) reported that the administration of T. terrestris extract (ETT) to tilapia larvae immersed in a dose of 0.01 g/L led to a male percentage of 92.75%. In the research by Mahadinata (2022) on the masculinization of guppy fish (Poecilia reticulata) with ETT through immersion of pregnant females, a male percentage of 87.78% was obtained with an immersion dose of 15 mg/L. A study published Kılınç et al. (2023), demonstrated that dietary supplements of T. terrestris significantly increased the affected the fertilization ability and spermatological characteristics of male rainbow trout (Oncorhynchus mykiss). Hassona et al. (2020) found that dietary supplementation with ETT specially 500 and 750 mg/kg diet significantly increased the proportion of male tilapia, improved growth rates, and enhanced survival rates compared to control groups. This study also examined how T. terrestris supplementation affects the blood plasma of male rainbow trout and it was found that the male rainbow trout fed with T. terrestris supplementation had the highest LH hormone value at 250 mg/kg. These descriptions showed that ETT could be used as a source of natural steroid hormones in sex direction processes. Therefore, this research aimed to evaluate the effect of ETT on growth performance, survival rate, and male percentage in freshwater crayfish through hormone feed and larval immersion.
Materials and Methods
The production of ETT included mixing 100 g of sieved, followed by weighing powder form with 1 liter of 90% ethanol in an Erlenmeyer flask and stirring for 2 hours in a water bath at 80. Subsequently, the solution was cooled and filtered using filter paper to obtain the filtrate, which was concentrated using a rotary vacuum evaporator at 0.8 ×g with a temperature of 45°C. The extract was placed in a dark bottle sealed with parafilm that had been pierced to allow any remaining ethanol to evaporate, and stored at –20°C (Do et al., 2013; Sasikumar et al., 2014). The concentrated extract was ready to be weighed and used according to the treatment dosage.
The maintenance container used was a CB45 measuring 54 × 36.5 × 28.5 cm3, totaling 30 units. Before use, the container was washed with soap to remove dirt adhering to the surface, rinsed with clean water, and dried for 24 hours. Initially, the container was covered with black plastic to create a dark environment, followed by filling with 20 L of water, and aeration was installed.
The female freshwater crayfish used was in the process of brooding eggs. The lobster larvae used were 14 days old, totaling 375 individuals with an average weight of 0.052 ± 0.07 g and a length of 11.16 ± 1.38 mm. Morphologically, the larvae were in good health (active swimming behavior, uniform coloration and responsiveness to external stimuli) and had complete organ systems.
Immersion was carried out five times, namely on days 0, 7, 14, 21, and 28. Initially, immersion container was filled with 2 liters of water, followed by the addition of the hormone MT and ETT according to the treatment doses. The water containing ETT was aerated for 12 hours to remove the remaining ethanol in the extract and larvae were immersed for 30 hours. After the immersion was completed, the larvae were transferred to the rearing container. The research design used in the study consists of five treatments with three replications. The treatments given were T1 (MT 2 mg/L), T2 (ETT 0 mg/L), T3 (ETT 10 mg/L), T4 (ETT 15 mg/L), and T5 (ETT 20 mg/L).
Treatment through feed was carried out by mixing hormones into artificial feed. The hormones to be used were dissolved in 90% ethanol at a rate of 50 mL/kg of feed and sprayed evenly on 1 kg of feed. Subsequently, the solution was stirred and air-dried at room temperature until all the alcohol was completely evaporated (6–12 hours). The feed was stored at –20°C and segregated into containers for each treatment, subsequently labeled. The feed was given three times a day for 40 days of maintenance. The research design used in the study consists of five treatments with three replications. The treatments given were N-Con: No treatment, P-Con: MT 50 ppm, T1: ETT 50 ppm, T2: ETT 100 ppm, and T3: ETT 200 ppm.
The sampling method for the testosterone (T) and estradiol (E2) level sampling, individuals were selected based on their identified sex within the sex-male ratio groups and their body weight. For the testosterone and estradiol level analysis of whole-body, two crayfish for each of the treatments at 60 days of rearing were used. The crayfish were homogenized in 500 µL of phosphate buffered saline solution (0.1 M NaCl, 0.002 M KCl, 0.01 M Na2HPO4, 0.002 M KH2PO4) using mortar. Homogenates were then vortexed for 20 s and centrifuges at 3,000×g for 10 min at 4°C to separate the supernatant containing T and E2 from coarse particles. The supernatant obtained is subsequently collected in microtubes and stored at a temperature of –20°C for further use in the determination of plasma T and E2 levels. T and E2 level was determined using a monoclonal antibody enzyme-linked immunosorbent assay (ELISA) quantification kit based on the principle of competitive binding. ELISA procedures and calculation following the manual book (catalogue number EIA-1887, DRG International, Springfield, NJ, USA). The measurement of T and E2 levels in this study was performed at the Agroindustry and Biomedical LaboratoryAgricultural Production, Puspitek-Serpong.
Blood glucose levels were analyzed using test crayfish samples at 60 days of rearing. Sampling involved collecting four juvenile crayfish from each experimental treatment group. The crayfish were anesthetized using ice. Sample preparation to extract the supernatant was carried out by homogenizing the test crayfish with phosphate-buffered saline solution at a 1:2 ratio until a uniform mixture was achieved. The samples were then centrifuged at 2,800 ×g at 4°C for 10 minutes. The plasma was separated, transferred into 1.5 mL tubes, and stored at –20°C until further analysis.
The analysis method employed was the Glucose Oxidase-Peroxidase Aminoantipyrine (GOD-PAP) technique. Test tubes were prepared for the blank, standard, and sample solutions. Each tube received 1,000 μL of GOD-PAP reagent solution. The standard tube was supplemented with 10 μL of GOD-PAP standard solution, the blank tube with 10 μL of distilled water, and the sample tube with 10 μL of blood plasma sample. All tubes were incubated for 10 minutes at 37°C. The absorbance of the standard, blank, and sample solutions was measured using a spectrophotometer at a wavelength of 500 nm. Blood glucose levels are calculated using the following equation:
The parameters observed were the percentage of male freshwater crayfish by comparing the number of males to the entire population, survival rate, glucose levels, testosterone, and estradiol hormone levels. Observation was also carried out on growth performance such as absolute length growth, absolute weight growth, and specific growth rate, including water quality. The parameters of male percentage, growth performance, and survival rate were analyzed using analysis of variance (ANOVA) at a 95% confidence level with SPSS version 22. When the treatment results differed significantly, further testing was conducted using Duncan’s test at a 95% confidence level, while water quality data was analyzed descriptively.
Results
The percentage of male freshwater crayfish in each soaking treatment varied significantly. The highest sex male ratio of crayfish was observed in the immersion method at T5 treatment (ETT 20 mg/L) with a value of 56.22 ±1.10%, followed by the 2 mg/L of MT (54.48 ± 2.07%), the 15 mg/L of ETT (53.19 ± 1.18%), the 10 mg/L of ETT (51.13 ± 3.99%), and the 0 mg/L of ETT (38.00 ± 2.96%). After statistical testing, the male percentage parameter showed significantly different results (p < 0.05), leading to further Duncan tests. The results showed that treatments T1 and T4 significantly differed from T2, but not varied to T3 and T5. In treatment T2, there was a significant difference from T1, T3, T4, and T5. Treatment T3 showed substantial variation from T2 and T5, but not significantly different from T1 and T4. In T5, there was a significant difference from T2 and T3, although not significantly different compared to T1 and T4. The results of the percentage of male freshwater crayfish given ETT by immersion method, as shown in Table 1.
As shown in Table 2, the percentage of male freshwater crayfish given ETT by oral method showed a varied pattern. The highest sex male ratio of freshwater crayfish was observed in the oral method at T3 treatment (ETT 200 ppm) with a value of 67.61 ± 8.02%, followed by the 50 ppm of MT (65.32 ± 9.39%), the 100 ppm of ETT (53.76 ± 1,63%), no treatment (37.46 ± 5.54%), and the 50 ppm of ETT (36.45 ± 6.98%). Based on the ANOVA results, the maintenance of ETT in feed with different doses significantly influenced the male percentage (p < 0.05), leading to further Duncan tests. The results showed that treatment E significantly differed from T2, N-Con, and T1 but not varied from treatment P-Con. Furthermore, in treatment P-Con, there was a significant difference between T1 and N-Con but not substantially varied from T2. N-Con significant difference was observed between N-Con and T1 in treatment T2, while K- showed no variation from T1.
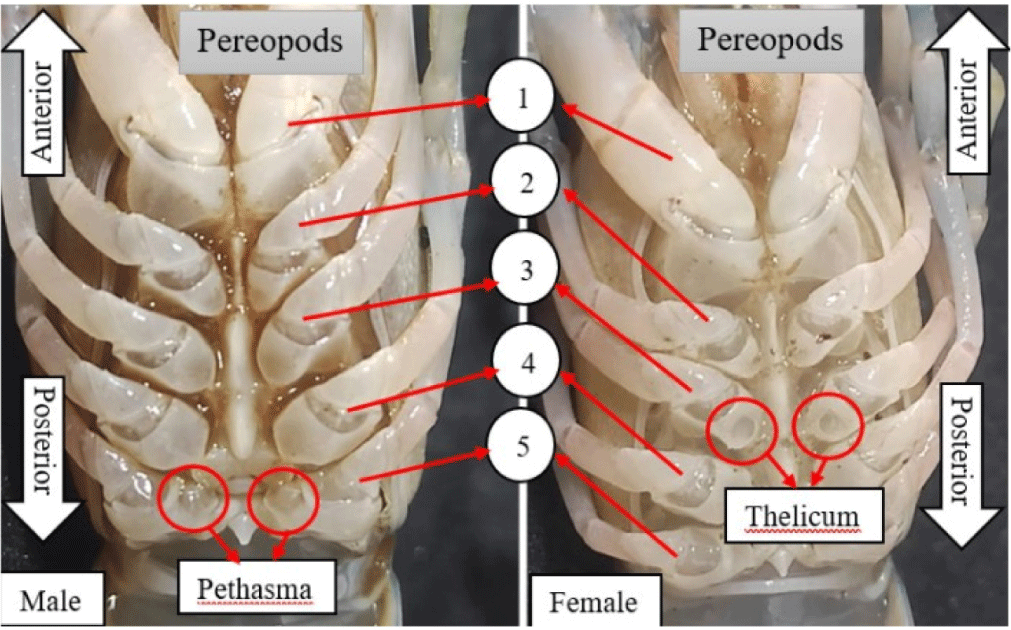
Gender in freshwater crayfish can be determined based on reproductive characteristics, such as color, shape, and other features. Sagi et al. (1996) stated that the primary distinguishing feature of gender was the presence of a protrusion on the walking legs, specifically in males on the fifth walking leg counted from the mouth, called petasma, while female was on the third walking leg called thelicum. Generally, male chelipeds are larger than female, with a red color at the tip, as shown in Fig. 1.
The testosterone levels in this research through immersion method ranged from 0.973 ± 0.03 to 1.028 ± 0.22 ng/mL and oral method ranged from 0.864 ± 0.05 to 0.929 ± 0.16 ng/mL. According to the ANOVA test, freshwater crayfish given different doses of ETT through immersion and oral methods showed no significant difference in testosterone levels (p > 0.05).
The estradiol levels in this research through immersion method ranged from 140.15–259.58 ng/mL and oral method ranged from 221.63–362.43 ng/mL. According to the results, samples given different doses of ETT showed no significant difference in estradiol levels (p > 0.05).
The growth performance was indicated by absolute length and weight as well as specific growth rate. Based on the analysis, the growth parameters of the sample given ETT with different doses in immersion and oral methods showed a relatively similar pattern. The absolute length growth in this research through immersion method ranged from 24.36 ± 1.01 to 25.68 ± 3.23 mm and oral method ranged from 23.81 ± 1.86 to 25.79 ± 1.39 mm, and then the absolute growth in this research through immersion method ranged from 1.177 ± 0.14 to 1.406 ± 0.17 g and oral method ranged from 1.33 ± 0.02 to 11.55 ± 0.18 g. According to the ANOVA, the maintenance of freshwater crayfish given ETT had no significant effect on absolute length, weight, and specific growth rate (p > 0.05). The growth performance results of samples treated with immersion and oral method are in Tables 1 and 2, respectively.
Survival rate parameters of freshwater crayfish given ETT at different doses in the immersion (Table 1) and oral method (Table 2) showed a relatively similar pattern. The survival rate in this research through immersion method ranged from 81.33 ± 10.0 to 88 ± 4.0% and oral method ranged from 64.00 ± 4.0 to 77.33 ± 12.2%. Based on the results of the ANOVA, the maintenance of samples that received ETT treatment showed no significant effect on survival rate (p > 0.05). This showed that the use of ETT did not have a negative impact on the survival of freshwater crayfish.
Glucose levels of freshwater crayfish given different doses of ETT in immersion and oral methods showed a relatively similar pattern. The glucose level in this research through immersion method ranged from 116.40 ± 29.7 to 164.68 ± 42.5 ng/mL and oral method ranged 116.64 ± 41.8 to 176.40 ± 36.9 ng/mL. Based on the ANOVA, the maintenance of samples that received ETT had a non-significant effect on glucose levels (p > 0.05).
Discussion
ETT contains protodioscin, which is an aphrodisiac, capable of increasing testosterone (Singh et al., 2023) (Supplementary Fig. S1 and Table S1). High levels of testosterone will direct the formation of male genitalia during the differentiation phase (Toyota et al., 2021). According to Ye et al. (2023) and Susanto et al. (2021), the mechanism of directing male sex in crustaceans is by increasing testosterone levels in the body and decreasing the estrogen to androgen ratio which causes the masculinization of secondary sexual characteristics. In this research, the percentage of male freshwater crayfish from both methods showed that with an increase in the dosage of the extract given, a higher percentage of males was produced. However, the percentage of male individuals did not reach 100% in both methods due to several factors. According to Dunn et al. (2020), factors that affected the success of masculinization included the dosage given, type of hormone, age, and timing. Sang et al. (2020) also reported that the administration of the appropriate dose of steroid hormones would inhibit the formation of one gonad, either ovaries or testes, thereby increasing the desired sex ratio percentage.
According to Susanto et al. (2023), the concentration of testosterone hormone in freshwater crayfish increased for each given dose. Administration of 2.5 mg/kg of bovine testis extract (BTE) mixed with feed could increase the concentration of testosterone hormone in the blood of female baung fish (Mystus nemurus) by 278.91 ± 66.76 pg/mL. The duration of BTE administration showed that for 30 days, there was an increase in the highest concentration of testosterone hormone to 254.38 ± 93.84 pg/ml. A study on the blue crab (Callinectes sapidus) reported testosterone levels in the range of 0.8–1.2 ng/mL (Wang et al., 2022). Similarly, research on the mud crab (Scylla paramamosain) found testosterone levels around 0.85–1.1 ng/mL (Knigge et al., 2021). Generally, hormones are organic chemicals, biologically active compounds produced by certain glands, tissues, or organs of animals and humans. These compounds work at low concentrations with specific modes of action, playing an important role in physiological regulation as activators or inhibitors of enzymes (Qaid & Abdoun, 2022). Testosterone and estradiol play crucial roles in the reproductive physiology of crustaceans. Testosterone is primarily involved in the development of male secondary sexual characteristics and spermatogenesis (Li et al., 2024).
The estradiol levels ranged from 140–362 ng/mL with both immersion and oral methods. According to Lewis et al. (2015), estradiol levels in the Norway lobster (Nephrops norvegicus) were reported to be around 100–250 ng/mL. Estradiol is an essential steroid, particularly for female crayfish passing through the vitellogenesis process (Gupta et al., 2021). The concentration of estradiol is the amount of estradiol hormone content in blood plasma to stimulate the liver to synthesize vitellogenin (Dahlia et al., 2023). The increase in testosterone and estradiol 17β hormone concentrations is highly influenced by species differences related to administration methods, doses, and hormone types. High testosterone hormone concentrations will provide adequate availability of estradiol 17β for the vitellogenesis process. This is because the testosterone hormone will be converted into estradiol 17β by the aromatase enzyme in granulosa cells (Roosta et al., 2022).
Fish farming is closely related to accelerating growth to reach the desired size or weight, thereby reducing maintenance time. In this study demonstrated that treatment with the ETT did not induce improvement in growth performance. Based to study by Matter et al. (2024), it found that while T. terrestris did not significantly enhance growth performance compared to control groups. This could be attributed to the antinutritional factors (hydrocyanic acid, phytate, nitrate, and oxalate) identified in TT leaves. According to Hidayat et al. (2013), growth is influenced by internal and external factors. Internal factors include age, gender, and genetic quality of fish, while external factors are related to the living environment such as physical, biological, and chemical conditions of the water. In this research, the administration of ETT did not show any effect on growth performance parameters due to the lack of content that could enhance or affect the development of freshwater crayfish. Similarly, Gharaei et al. (2020) stated that the administration of ETT did not significantly affect the growth parameters of zebrafish. According to Cui et al. (2022), fish growth could be influenced by internal factors such as gender, age, and fish genetics, as well as external factors such as water chemistry and food.
Survival rate is an important parameter in fish activities, which is influenced by several factors such as hormone toxicity levels, feed quantity, stocking density, and water quality (Onxayvieng et al., 2021). This study indicates that the most effective treatments for producing male crayfish were 20 mg/L immersion and 200 mg/kg oral administration. These levels align with the hormonal sensitivity of juvenile crayfish during critical developmental stages. According to Banh et al. (2021), sufficient exposure to exogenous steroids can effectively influence sexual differentiation. However, differences in ETT sensitivity may exist among species due to variations in hormonal pathways and physiological responses. The administration of excessively high doses can lead to death, intersex individuals, or inhibited gonad development to sterility. Indratmoko et al. (2023) stated that the dominant component in ETT was protodioscin, which had no toxic effects and was considered safe for use. Additionally, one of the causes of mortality in crustaceans is cannibalism. According to Kelly et al. (2023), freshwater crayfish showed cannibalistic behavior, particularly at a young age and high density. In this research, cannibalism could be observed through the presence of crayfish with deformities (incomplete limbs) and the remains of dead lobsters.
The measurement of blood glucose levels shows the stress levels experienced by crayfish during maintenance. In this research, glucose levels were not affected by the administration of ETT because this extract did not contain toxic substances. An increase in glucose levels in freshwater crayfish was used as a secondary marker of stress response (Costantini et al., 2022). Generally, freshwater crayfish are known as one of the species that are strong and resilient in handling stress (Oellermann et al., 2022). This research showed that the blood glucose levels of larvae were still within normal limits. The glucose levels in crustaceans under normal conditions generally do not exceed 150 mg/L (Mahasri et al., 2022). Higher glucose levels indicate an increase in stress levels (Odhiambo et al., 2020). According to Hattori et al. (2020), changes in environmental conditions would cause stress and affect the main stress response related to cortisol. High cortisol levels can also inhibit aromatase genes, leading to an increase in 11-ketotestosterone levels, which is capable of inducing the development of male sex organs (Zhou et al., 2021). In conclusion, this research showed that the administration of ETT only affected testosterone but did not influence glucose levels.
We recognize the importance of addressing the study’s limitations and potential confounding factors to enhance the robustness of the discussion. Some limitations include the relatively small sample size and the specific environmental conditions under which the trials were conducted, which might limit the generalizability of the findings. Furthermore, the potential influence of unmeasured variables, such as genetic variation among individuals, could also have affected the results.
To emphasize the novelty of our findings, we highlight that this study provides new insights into the effects of ETT treatment on sex male ratio in freshwater crayfish. These findings contribute to a deeper understanding of how targeted interventions can optimize freshwater crayfish male production in aquaculture practices.
